Abstract
Thrombocytopenia is common among sick neonates. Certain groups of thrombocytopenic adults respond favorably to the administration of recombinant thrombopoietin or to pegylated recombinant human megakaryocyte growth and development factor (PEG-rHuMGDF), a recombinant human polypeptide that contains the receptor-binding N-terminal domain of thrombopoietin. The effectiveness and safety of such treatment in neonates, however, have not been reported. The purpose of the present study was to determine the biologic activity and safety of PEG-rHuMGDF administration to newborn rhesus monkeys. Eight monkeys were divided into four groups and treated subcutaneously with 0.00, 0.25, 1.00, or 2.50 μg/kg once daily for 7 d. Complete blood counts, serum chemistries, clotting panels, and MGDF levels were followed serially, and hematopoietic progenitor cell assays were performed on bone marrow aspirates before the first dose and again on d 8. Pharmacokinetic evaluations were performed on the animals that received the highest dose of PEG-rHuMGDF. All monkeys had normal growth during the study period, and all chemistries, clotting studies, and blood pressure measurements were normal. The peak serum MGDF concentration occurred at 3 h, and the half-life was 8.4 to 13.0 h. As in adult rhesus monkeys, platelet counts in the treated neonates began to rise on d 6, peaked on d 11, and returned to baseline by d 23. The two highest doses generated an 8- to 12-fold increase in platelets, whereas those treated with 0.25 μg/kg had a 6-fold increase. Other hematologic parameters measured were unaffected. Thus, newborn monkeys responded to doses of PEG-rHuMGDF that were similar to or smaller than (per kilogram body weight) those that are effective in adult animals and did so without obvious short-term toxicity.
Similar content being viewed by others
Main
Tpo is the recently discovered physiologic stimulator of platelet production (1). Since 1994 when Tpo was first purified and cloned (2, 3), multiple in vitro and in vivo studies have been conducted using the recombinant molecule. In vitro, rTpo has direct proliferative and differentiative activities on human megakaryocyte progenitors (4–6). In vivo, rTpo administered to mice and nonhuman primates induces significant thrombocytosis and decreases or completely prevents the thrombocytopenia resulting from myelosuppressive chemotherapy (7–9). Recent clinical trials in adult humans showed similar responses (10–13).
Thrombocytopenia is a frequent problem in sick neonates, affecting 22 to 35% of all babies admitted to NICU (14, 15). In most cases of neonatal thrombocytopenia, the etiology is unknown, but recent evidence suggests that in many patients the kinetic mechanism is reduced platelet production with a decreased megakaryocyte mass (16). Moreover, studies by Murray et al. (17) suggest that an inadequate elevation of Tpo levels may complicate some cases of thrombocytopenia in preterm infants. In most of these cases, the only available treatment is to repeatedly transfuse platelets, exposing the infants to many known risks. For example, although blood donors are screened extensively, contamination with bacterial or viral pathogens such as cytomegalovirus can occur. In addition, multiple transfusions are frequently necessary, thus exposing the neonate to multiple donors and increasing the potential for transfusion reactions, graft-versus-host disease, and platelet refractoriness.
Morbidity among thrombocytopenic neonates, whether or not they receive platelet transfusions, is among the highest of any neonatal patient group encountered (18). The high incidence of thrombocytopenia in patients in the NICU and the poor outcome associated with this condition indicate that this is a serious public health problem and suggest that alternate methods of treatment should be pursued.
Megakaryocyte progenitors from umbilical cord blood (19) and from peripheral blood of preterm and term infants (17) are responsive to rTpo in vitro, but it is unknown whether they respond in vivo and whether any age-related differences exist in the thrombopoietic activity, pharmacokinetics, or toxicity of rTpo. Failure to recognize such age-related differences has historically represented a significant problem. Such was the case with Epo, initially believed to be ineffective for the treatment of anemia of prematurity on the basis of clinical trials that used “adult-like” doses of rEpo per kilogram of body weight to treat preterm neonates (20). In 1990, a study comparing the erythropoietic responses to rEpo in adult versus infant rhesus monkeys showed that rEpo had very different pharmacokinetic properties in infants (21). Specifically, neonates had a much larger volume of distribution of rEpo and more rapid clearance and, therefore, required substantially higher doses of rEpo per kilogram of body weight than adults to achieve an equivalent biologic effect. That study was an important step toward the successful use of higher doses of rEpo to treat the anemia of prematurity.
Because Tpo is a molecule with significant homology to Epo (1), the present study was designed to determine whether age-related differences are significant for rTpo. For the study, PEG-rHuMGDF, a recombinant human polypeptide that contains the receptor-binding N-terminal domain of Tpo (22) and that has been the formulation chosen for most human and animal studies, was used. We hypothesized that 1) newborn rhesus monkeys will respond to PEG-rHuMGDF with an increase in platelet counts in a manner similar to that reported in adult monkeys and adult humans;2) newborn rhesus monkeys treated with therapeutic doses of PEG-rHuMGDF will not experience adverse effects; and 3) the pharmacokinetics and pharmacodynamics of PEG-rHuMGDF in newborn rhesus monkeys will differ from those measured in adult monkeys. To test these hypotheses, we treated eight newborn rhesus monkeys with various doses of PEG-rHuMGDF for 7 d and evaluated their responses.
METHODS
Animals
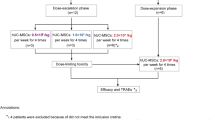
Normally cycling adult female rhesus macaques (Macaca mulatta) with a history of prior pregnancy were bred and identified as pregnant according to established methods (23). All procedures used within the study conformed to the requirements of the Animal Welfare Act, and study protocols were approved before implementation by the Institutional Animal Use and Care Administrative Advisory Committee at the University of California at Davis. Activities related to animal care (diet, housing, breeding) were performed according to standard California Regional Primate Research Center (CRPRC) operating procedures.
Monkeys were delivered by cesarean section near term (159 ± 1 d; term 165 ± 10 d) with collection of cord blood (6 mL) at the time of delivery. Simian Apgar scores were assessed at birth, and body and placental weights and morphometric evaluations were performed (24). Neonates that delivered spontaneously before scheduled cesarean sections were removed from the dams once detected and within approximately 8 h of when delivery occurred. All neonates were placed in the nursery and hand-reared for postnatal studies.
Test and Control Articles
PEG-rHuMGDF and the placebo were supplied by Amgen, Inc. (Thousand Oaks, CA). Dilutions were made each day for once daily subcutaneous administration for 7-d duration in a 0.25-mL volume.
Placebo/vehicle.
The vehicle was supplied in 10-mL vials. BSA was added to make a BSA:placebo solution (6 mL placebo + 0.6 mL 2.5% BSA). The BSA:placebo solution was used for all dilutions and for treating neonates in the control group.
PEG-rHuMGDF.
rHuMGDF (Amgen, Inc.) is a nonglycosylated polypeptide produced in Escherichia coli transfected with a plasmid containing cDNA that encodes for a region encompassing the Epo-like amino terminal domain of human Tpo. After extraction, refolding, and purification, this truncated protein is conjugated with polyethylene glycol (PEG-rHuMGDF), and further purified. PEG-rHuMGDF was kindly supplied by Amgen, Inc. in 2-mL vials (2.5 mg/mL). For the high dose (2.5 μg/kg), 20 μL stock rHuMGDF was added to 980 μL BSA:placebo. Thus, for a 500-g monkey, 0.25 mL of this solution was administered (1.25 μg). For the middose (1.0 μg/kg), for a 500 g monkey, 0.1 mL diluted rHuMGDF (see high dose) + 0.15 mL BSA:placebo (0.5 μg) was administered. For the low dose (0.25 μg/kg), for a 500-g monkey, 0.025 mL diluted rHuMGDF + 0.225 mL BSA:placebo (0.125 μg) was administered.
Neonatal Treatment and Evaluation
Treatment.
Neonates were assigned to the study on a rotational basis. Each neonate was administered PEG-rHuMGDF subcutaneously once daily at 0.00, 0.25, 1.00, or 2.50 μg·kg−1·d−1. Two animals were treated with each dose. The doses chosen were based on studies performed in adult rhesus monkeys (L. Roskos, Amgen, Inc., personal communication, 1998) in which a saturable response profile was observed, with similar platelet increases at doses of 2.50, 7.50, and 25.00 μg·kg−1·d−1. This suggested that the concentration-response profile would be well characterized using doses between 0.25 and 2.50 μg·kg−1·d−1.
Growth and development.
Body weights were determined at birth and daily throughout the study period. Morphometric evaluations were conducted on postnatal d 1, 8, and 28.
Blood samples.
Peripheral blood samples were collected from a femoral vessel in hand-restrained (unanesthetized) infants. Samples were used for complete blood counts (study d 2, 4, and 6 and then twice weekly until postnatal d 28), clotting and chemistry panels (birth and d 8), and rHuMGDF levels (birth, d 4 and 8). The chemistry panels included chloride, sodium, potassium, calcium, phosphorus, albumin, BUN, creatinine, glucose, total protein, alanine aminotransferase, aspartate aminotransferase, alkaline phosphatase, total bilirubin, cholesterol, and triglycerides.
Hematopoietic progenitor cell assays.
Bone marrow was collected for hematopoietic progenitor cell assays from each monkey aseptically under local anesthesia (1% lidocaine) on the day of birth (before treatment) and again at the end of the treatment period (postnatal d 8). For the quantification of pluripotent CFU-Mix, CFU-GM, and BFU-E and CFU-E progenitors, nucleated bone marrow cells (0.2 × 105 cells/mL) were cultured in duplicate in Methocult GF + H4435 (StemCell Technologies, Inc., Vancouver, Canada) and assayed in parallel by using standard techniques (25). Quantitation of the individual colonies was performed after a standard incubation period (10 d).
For the quantification of primitive (BFU-MK) and mature (CFU-MK) megakaryocytic progenitors, the light density cells were resuspended in Iscove's modified Dulbecco's medium (MDM) and subsequently cultured in a serum-free semisolid growth medium containing 10 ng/mL each of rhIL-3 and rhIL-6 and 50 ng/mL of rhTpo (MegaCult-C, StemCell Technologies, Inc., Vancouver, Canada). The final culture mixture (0.75 mL) was dispensed into each of the two wells of a previously marked double-chamber slide (Permanox, Nalge Nunc International, Naperville, IL). After a 10-d incubation period, the slides were dehydrated and fixed using a 1:3 methanol:acetone solution. Subsequently, immunocytochemical staining was performed using mouse anti-human GPIIb/IIIa (StemCell Technologies, Inc., Vancouver, Canada) as the primary antibody. After the staining procedure, each slide was entirely scanned using a 5X objective lens in a regular light microscope, and all colonies (megakaryocytic and nonmegakaryocytic) were counted. The megakaryocytes, which express GPIIb/IIIa (CD41), were easily recognized by their pink color after the staining.
Blood pressure.
Blood pressure was assessed at birth (before treatment), every other day during the treatment period, and once posttreatment (d 9), using a Dinamap Vital Signs monitor.
Pharmacokinetics.
Blood samples were collected for rHuMGDF levels from all animals at birth, predose on d 4, and on d 8 (24 h after the last dose). Neonates in the high-dose group (2.50 μg·kg−1·d−1) had additional blood samples collected on the seventh day of treatment at 1, 3, 8, 12, and 24 h postdose. The serum was frozen at ≤−70°C until analysis. The concentrations of rHuMGDF were determined by ELISA (Amgen, Inc., Thousand Oaks, CA). The lower limit of quantification of the assay used was 0.167 ng/mL.
Statistical Analysis
Mean and SD of each hematologic parameter and of rHuMGDF serum concentrations were calculated from the animals in each dose group for each time point available. Pharmacokinetic parameters (Cmax, Tmax, AUC0–24h, CL/F, and T1/2) were calculated using WinNonlin v. 1.1. Tests for differences in treatment means were carried out using standard ANOVA methods.
RESULTS
Neonatal outcome.
There were no adverse effects detected during the study period. All infants showed normal growth and activity patterns. There were no abnormalities detected in chemistry parameters or blood pressure measurements. All such values were within normal limits for infants in this age group when compared with concurrent and historical controls (n = 40).
Hematologic parameters.
Platelet counts in the treated neonates increased (Fig. 1), with the two highest doses (1.00 and 2.50 μg·kg−1·d−1) generating an 8- to 12-fold increase from the baseline counts. There were no significant differences detected between the platelet counts obtained with the two highest doses. Newborn monkeys treated with 0.25 μg·kg−1·d−1 had a 6-fold increase in platelet count. In all treated animals, the increase in blood platelet concentration was first observed on d 6, reached its peak on d 11, and returned to baseline by d 23. The placebo-treated animals had the expected increase in platelet concentrations over the first 2 wk of life and returned to baseline by d 23 (Tarantal, unpublished data). However, there was a significant difference between the mean peak platelet count of the treated animals versus that of the controls (3457 ± 633 versus 1032 ± 104 × 109/L, mean ± SD, p < 0.03). All eight animals also had platelet counts performed 10 mo after the study, and all were within normal limits (range 467 to 738 × 109/L).
Dose-response of PEG-rHuMGDF on the blood platelet concentration in newborn rhesus monkeys. The animals received daily subcutaneous injections of placebo or PEG-rHuMGDF at doses of 0.25, 1.00, or 2.50 μg·kg−1·d−1 for 7 d. Each line represents the average platelet counts of the two monkeys in each treatment group, sampled on study d 2, 4, and 6 and then twice weekly until d 28. The peripheral platelet count increased on d 6 of treatment, peaked on d 11, and returned to baseline by d 23. The two higher doses generated similar increases in the platelet count.
In all MGDF treated animals, the mean platelet volume decreased as the platelet count increased and returned to baseline about the same time as the platelet counts (Fig. 2). A similar change, but of less magnitude, was observed in the placebo-treated infants. There was no correlation between the change in mean platelet volume and the dose of PEG-rHuMGDF used, and the difference between the treated (−14.1%) and control animals (−11.6%) was not significant.
Effects of treatment with PEG-rHuMGDF on the mean platelet volume (MPV) in newborn rhesus monkeys. Each line represents the average MPV of the two monkeys in each treatment group, sampled as described in Figure 1. The MPV decreased in the treated animals by approximately 15% and normalized at the time that the platelet count returned to baseline. There was no correlation between the change in MPV and the dose of PEG-rHuMGDF administered.
The administration of PEG-rHuMGDF did not affect any other hematologic parameter measured. Hb, hematocrit, and mean corpuscular volume decreased in all animals, and no differences were observed between the treated and vehicle-control groups. Similarly, the white blood cell counts and differential counts were not affected by PEG-rHuMGDF treatment.
Progenitor assays.
The average number of pluripotent, erythroid, granulocytic, and megakaryocytic colonies on d 1 and at the end of the treatment period (d 8) is shown in Table 1. We observed a trend toward an increase in all progenitors after treatment with the higher doses of PEG-rHuMGDF. No statistical testing was performed due to the small sample size in each treatment group.
Pharmacokinetics.
PEG-rHuMGDF levels were below the limits of quantification in all animals on d 1 (pretreatment) and at all time points in the vehicle-control animals. Pretreatment concentrations obtained after 4 d of dosing remained undetectable in the animals receiving 0.25 μg·kg−1·d−1 but increased in a dose-dependent manner in the animals treated with 1.00 and 2.50 μg·kg−1·d−1 of PEG-rHuMGDF (means of 0.794 ng/mL and 1.179 ng/mL, respectively). Concentrations measured 24 h after the last dose (d 8) were 2–3-fold lower in the animals receiving the two higher doses when compared with the trough levels obtained on d 4. Among the animals treated with 0.25 μg·kg−1·d−1, whose levels had been below detectable limits on d 4, one had a level of 0.339 ng/mL on d 8 and the other remained undetectable.
Pharmacokinetic profiles were determined after the seventh dose of 2.50 μg·kg−1·d−1 (Fig. 3). The peak serum concentration (mean of 2.14 ng/mL) was observed at 3 h in both animals. The AUC0–24 h varied significantly from one animal to the other, with one having an AUC of 31.3 ng·h−1·mL−1 and the other 16.1 ng·h−1·mL−1. The half-life for the drug was 8.4 h in one and 13.0 h in the other.
DISCUSSION
Neonates in intensive care units have a high incidence of thrombocytopenia (26). For example, during 1997 at the University of Florida, Shands Children's Hospital, 69 of our non-ECMO (extracorporeal membrane oxygenator) NICU patients (8% of our annual admissions) received at least one platelet transfusion, and 37 (54%) of those received two or more platelet transfusions (mean ± SD, 5.1 ± 4.3; median, 4.0). It is unknown whether rTpo administration could be used to decrease the need for platelet transfusions in some thrombocytopenic neonates, but there is evidence that inadequate Tpo production can contribute to the development of thrombocytopenia in neonates. Previous studies by our group indicated that Tpo and its receptor (c-mpl) are expressed by the human fetus at about the same time platelet production first appears (27) and that certain groups of thrombocytopenic preterm neonates do not increase their plasma Tpo concentrations as do thrombocytopenic adults (28). As a step toward determining whether thrombocytopenic neonates would respond to rTpo in vivo, we performed the present study in which we evaluated the thrombopoietic effects, pharmacokinetics, and safety of PEG-rHuMGDF administered to newborn rhesus monkeys.
Eight monkeys were divided into four groups. Three groups were treated with PEG-rHuMGDF for 7 d, and one group received a placebo identical to the vehicle of the drug. We observed that, like adult monkeys and adult humans, the newborn monkeys experienced a significant dose-dependent increase in their platelet counts. However, the use of doses higher than 1.00 μg·kg−1·d−1 did not result in further increments in platelet counts, most likely reflecting a maximal level of stimulation of the process of megakaryocyte proliferation and maturation. A previous study evaluating the effects of 10 d of nonpegylated rHuMGDF in adult rhesus monkeys (29) showed a maximal response to a dose of 25 μg·kg−1·d−1. Because the pegylation of rHu-MGDF results in a 10-fold increase of activity (30), this would suggest that 2.50 μg·kg−1·d−1 of PEG-rHuMGDF should generate a maximal level of stimulation in adult rhesus monkeys. In fact, studies performed in adult rhesus monkeys treated with PEG-rHuMGDF (L. Roskos, Amgen, Inc., personal communication, 1998) demonstrated similar platelet increases at doses of 2.50, 7.50, and 25.00 μg·kg−1·d−1, indicating a saturable response profile. Harker et al. (30) evaluated the dose-response effects of PEG-rHuMGDF on platelet production in 22 adult baboons treated for 28 d. They found that, within the range of doses studied (0.05, 0.10, 0.50, and 2.50 μg·kg−1·d−1), the increase in platelet count was directly related to the logarithm of the dose. They did not test, however, whether higher doses would have induced a further increment in platelet count.
When evaluating the magnitude of the response to PEG-rHuMGDF, the physiologic changes in the platelet counts observed in rhesus monkeys in the first 2 wk of life need to be considered. Although newborn monkeys treated with 0.25 μg·kg−1·d−1 experienced a 6-fold increase from their platelet counts at birth and those receiving higher doses showed an 8- to 12- fold increase, the placebo-treated infants doubled their platelet concentrations at around 2 wk of life. Consequently, the MGDF-induced increase in platelet counts was 3-fold in the low-dose group, and 4- to 6-fold in the intermediate- and high-dose groups. This degree of response is similar to that observed in adult nonhuman primates (29, 30) and in adult humans with advanced cancer receiving comparable doses of PEG-rHuMGDF (0.3 and 1.0 μg·kg−1·d−1) (10). The peak platelet counts observed in newborn monkeys (close to 4000 × 109/L), however, are significantly higher than those achieved in adult monkeys (usually <3000 × 109/L) or humans (<2000 × 109/L) undergoing thrombopoietic stimulation. This is not surprising, considering that the normal platelet count in 2-wk old rhesus monkeys is 850 ± 71 × 109/L (Tarantal, unpublished data).
We found that the pattern of response to PEG-rHuMGDF in newborn monkeys was similar to that in multiple reports of adult animals and adult humans (22). Specifically, platelet counts begin to increase after 3 to 6 d, peak at approximately 2 wk, and return to baseline within 2 wk after discontinuing therapy. This pattern indicates that in newborn as well as adult monkeys, rTpo does not cause an immediate release of platelets from megakaryocytes and does not accelerate the fragmentation of pro-platelet processes. Instead, its effects depend on stimulation of the production and maturation of megakaryocytes, which accounts for its prolonged biologic action beyond the end of therapy.
In addition to its thrombopoietic effects, we evaluated the pharmacokinetics of PEG-rHuMGDF in newborn monkeys. On the first day of life, before initiating treatment, the levels were below quantifiable limits for our ELISA (<0.167 ng/mL) in all animals. Similarly to adult monkeys (30), serum trough levels measured on the fourth day of treatment showed a dose-dependent increase in the infants that received the two higher doses and remained undetectable in the placebo and low-dose–treated monkeys. The trough levels measured in the two animals treated with 2.50 μg·kg−1·d−1 (1.32 and 1.04 ng/mL) were within the range of the average trough reported by Harker et al. (30) in adult baboons receiving the same dose (1.52 ± 0.51 ng/mL). Trough PEG-rHuMGDF concentrations measured on d 8 (24 h after the last dose) were significantly lower than on d 4 in the monkeys treated with the higher doses. Because serum levels of Tpo are regulated to a large extent by its binding to its specific receptor (c-mpl) in platelets and megakaryocytes (31–33), this decrease most likely reflects the increased number of receptors available as the platelet count and megakaryocyte mass increased. Although we did not test for the development of anti-Tpo antibodies, platelet counts performed 10 mo after the treatment period were within normal limits for all animals.
Pharmacokinetic profiles determined in the high-dose group after the seventh dose showed that the peak serum concentration of PEG-rHuMGDF was obtained 3 h after subcutaneous dosing, a time comparable to that measured in adult rhesus monkeys (4 ± 2 h). Similarly, the mean half-life for the drug in the newborn monkeys (10.7 h) was within the normal range for adult rhesus monkeys (9.4 ± 1.8 h) (L. Roskos, Amgen, Inc., personal communication, 1998).
To evaluate the effects of PEG-rHuMGDF on the hematopoietic progenitors of newborn monkeys, we quantified the pluripotent, megakaryocytic, granulocytic, and erythroid progenitors in marrow before and at the end of the treatment. The treated animals showed a trend toward a higher number of progenitors of all types, although the small sample size in each group precluded statistical testing. The effects of rTpo or its derivatives on the hematopoietic progenitors of adult humans and adult primates have been controversial. Whereas some investigators have reported an increase in the concentration of all bone marrow progenitors (11), others have observed no change (34).
Although the number of newborn monkeys evaluated in our study is small, the results suggest that neonates respond to doses of PEG-rHuMGDF comparable (per kilogram of body weight) to those effective in adult animals. Thus, in contrast with rEpo, smaller doses of PEG-rHuMGDF than are needed for adults may achieve the desired effect in neonates. This sensitivity is likely due to an increased responsiveness of the megakaryocyte progenitors of neonates to PEG-rHuMGDF. In addition, the pattern of response is very similar to that observed in adults, providing a prolonged effect. Although many questions remain, our findings suggest that human neonates may respond to PEG-rHuMGDF (or other Tpo-like molecules) in a manner similar to adults, and thus the benefits and risks of this treatment could be tested as an alternative to platelet transfusions for the management of thrombocytopenia in neonates. Further studies aimed at better defining the natural history and kinetic mechanisms responsible for the different varieties of neonatal thrombocytopenia are necessary to precisely determine which thrombocytopenic neonates would be the best candidates for such therapy.
Shortly after the completion of this study, clinical trials involving the use of PEG-rHuMGDF were interrupted. However, phase II and III clinical trials involving the full-length rTpo molecule are ongoing, and some laboratories are developing new peptides with similar thrombopoietic activity (35, 36). The availability of rTpo or other Tpo-mimetic peptides for clinical use in the very near future is certain.
Abbreviations
- rTpo:
-
recombinant thrombopoietin
- PEG-rHuMGDF:
-
pegylated recombinant human megakaryocyte growth and development factor
- NICU:
-
neonatal intensive care unit
- rEpo:
-
recombinant erythropoietin
- CFU-Mix:
-
pluripotent hematopoietic progenitor
- CFU-GM:
-
granulocyte-macrophage colony-forming unit
- BFU-E:
-
burst-forming unit-erythroid
- CFU-E:
-
colony-forming unit-erythroid
- AUC:
-
area under the serum concentration versus time curve
References
Kaushansky K 1995 Thrombopoietin: the primary regulator of platelet production. Blood 86: 419–431
de Sauvage FJ, Hass PE, Spencer SD, Malloy BE, Gurney AL, Spencer SA, Darbonne WC, Henzel WJ, Wong SC, Kuang WJ, Oles KJ, Hultgren B, Solberg LA Jr, Goeddel DV, Eaton DL 1994 Stimulation of megakaryocytopoiesis and thrombopoiesis by the c-Mpl ligand. Nature 369: 533–538
Lok S, Kaushansky K, Holly RD, Kuijper Jl, Lofton-Day CE, Oort PJ, Grant FJ, Heipel MD, Burkhead SK, Kramer JM, Bell LA, Sprecher CA, Blumberg H, Johnson R, Prunkard D, Hing AFT, Mathewes SL, Bailey MC, Forstrom JW, Buddle MM, Osborn SG, Evans SJ, Sheppard PO, Presnell SR, O'Hara PJ, Hagen FS, Roth GJ, Hagen Roth GJ, Foster DC 1994 Cloning and expression of murine thrombopoietin cDNA and stimulation of platelet production in vivo. Nature 369: 565–568
Zeigler FC, de Sauvage F, Widmer HR, Keller GA, Donahue C, Schreiber RD, Malloy B, Hass P, Eaton D, Matthews W 1994 In vitro megakaryocytopoietic and thrombopoietic activity of c-mpl ligand (TPO) on purified murine hematopoietic stem cells. Blood 84: 4045–4052
Banu N, Wang JF, Deng B, Groopman JE, Avraham H 1995 Modulation of megakaryocytopoiesis by thrombopoietin: the c-Mpl ligand. Blood 86: 1331–1338
Debili N, Wendling F, Katz A, Guichard J, Breton-Gorius J, Hunt P, Vainchenker W 1995 The Mpl ligand or thrombopoietin or megakaryocyte growth and differentiative factor has both direct proliferative and differentiative activities on human megakaryocyte progenitors. Blood 86: 2516–2525
Ulich TR, del Castillo J, Yin S, Swift S, Padilla D, Senaldi G, Bennett L, Shutter J, Bogenberger J, Sun D, Samal B, Shimamoto G, Lee R, Steinbrink R, Boone T, Sheridan WT, Hunt P 1995 Megakaryocyte growth and development factor ameliorates carboplatin-induced thrombocytopenia in mice. Blood 86: 971–976
Harker LA, Hunt P, Marzec UM, Kelly AB, Tomer A, Hanson SR, Stead RB 1996 Regulation of platelet production and function by megakaryocyte growth and development factor in nonhuman primates. Blood 87: 1833–1844
Grossmann A, Lenox J, Ren HP, Humes JM, Forstrom JW, Kaushansky K, Sprugel KH 1996 Thrombopoietin accelerates platelet, red blood cell, and neutrophil recovery in myelosuppressed mice. Exp Hematol 24: 1238–1246
Basser RL, Rasko JEJ, Clarke K, Cebon J, Green MD, Hussein S, Alt C, Menchaca D, Tomita D, Marty J, Fox RM, Begley CG 1996 Thrombopoietic effects of pegylated recombinant human megakaryocyte growth and development factor (PEG-rHuMGDF) in patients with advanced cancer. Lancet 348: 1279–1281
Vadhan-Raj S, Murray LJ, Bueso-Ramos C, Patel S, Reddy SP, Hoots WK, Johnston T, Papadopolous NE, Hittelman WN, Johnston DA, Yang TA, Paton VE, Cohen RL, Hellmann SD, Benjamin RS, Broxmeyer HE 1997 Stimulation of megakaryocyte and platelet production by a single dose of recombinant human thrombopoietin in patients with cancer. Ann Intern Med 126: 673–681
Fanucchi M, Glaspy J, Crawford J, Garst J, Figlin R, Sheridan W, Menchaca D, Tomita D, Ozer H, Harker L 1997 Effects of polyethylene glycol-conjugated recombinant human megakaryocyte growth and development factor on platelet counts after chemotherapy for lung cancer. N Engl J Med 336: 404–409
Basser RL, Rasko JEJ, Clarke K, Cebon J, Green MD, Grigg AP, Zalcberg J, Cohen B, O'Byrne J, Menchaca DM, Fox RM, Begley CG 1997 Randomized, blinded, placebo-controlled phase I trial of pegylated recombinant human megakaryocyte growth and development factor with filgrastim after dose-intensive chemotherapy in patients with advanced cancer. Blood 89: 3118–3128
Mehta P, Vasa R, Neumann L, Karpatkin M 1980 Thrombocytopenia in the high-risk infant. J Pediatr 97: 791–794
Castle V, Andrew M, Kelton J, Giron D, Johnston M, Carter C 1986 Frequency and mechanism of neonatal thrombocytopenia. J Pediatr 108: 749–755
Murray NA, Roberts IAG 1996 Circulating megakaryocytes and their progenitors in early thrombocytopenia in preterm neonates. Pediatr Res 40: 112–119
Murray NA, Watts TL, Roberts IAG 1998 Endogenous thrombopoietin levels and effect of recombinant human thrombopoietin on megakaryocyte precursors in term and preterm babies. Pediatr Res 43: 148–151
Andrew M, Castle V, Saigal S, Carter C, Kelton JG 1987 Clinical impact of neonatal thrombocytopenia. J Pediatr 110: 457–464
Nishihira H, Toyoda Y, Miyazaki H, Kigasawa H, Ohsaki E 1996 Growth of macroscopic human megakaryocyte colonies from cord blood in culture with recombinant human thrombopoietin (c-mpl ligand) and the effects of gestational age on frequency of colonies. Br J Haematol 92: 23–28
Sola MC, Christensen RD 1997 Use of hematopoietic growth factors in the neonatal intensive care unit. J Intensive Care Med 12: 187–205
George JW, Bracco CA, Shannon KM, Davis J, Smith IL, Phibbs RH, Hendrickx AG 1990 Age-related differences in erythropoietic response to recombinant human erythropoietin: comparison in adult and infant rhesus monkeys. Pediatr Res 28: 567–571
Kaushansky K 1998 Thrombopoietin. N Engl J Med 339: 746–754
Tarantal AF, Hendrickx AG 1988 The use of ultrasound for early pregnancy detection in the rhesus and cynomolgus macaque. J Med Primatol 17: 105–112
Tarantal AF, Hendrickx AG 1989 Evaluation of the bioeffects of prenatal ultrasound exposure in the cynomolgus macaque (Macaca fascicularis). Teratology 39: 137–147
Tarantal AF, Gargosky SE, O'Brian WD, Hendrickx AG 1995 Hematologic and growth-related effects of frequent prenatal ultrasound exposure in the long-tailed macaque (Macaca fascicularis). Ultrasound Med Biol 21: 1073–1081
Andrew M, Kelton J 1984 Neonatal thrombocytopenia. Clinic Perinatol 11: 359–391
Sola MC, Juul SE, Meng YG, Garg S, Sims P, Calhoun DA, Dame JB, Christensen RD 1999 Thrombopoietin (Tpo) in the fetus and neonate: Tpo concentrations in preterm and term neonates, and organ distribution of Tpo and its receptor (c-mpl) during human fetal development. Early Hum Dev 53: 239–250
Sola MC, Calhoun DA, Hutson AD, Christensen RD 1999 Plasma thrombopoietin concentrations in thrombocytopenic and non-thrombocytopenic patients in a neonatal intensive care unit. Br J Haematol 104: 90–92
Farese AM, Hunt P, Boone T, MacVittie T 1996 Recombinant human megakaryocyte growth and development factor stimulates thrombocytopoiesis in normal nonhuman primates. Blood 86: 54–59
Harker LA, Marzec UM, Hunt P, Kelly AB, Tomer A, Cheung E, Hanson SR, Stead RB 1996 Dose-response effects of pegylated human megakaryocyte growth and development factor on platelet production and function in nonhuman primates. Blood 88: 511–521
Broudy VC, Lin NL, Sabath DF, Papayannopoulou T, Kaushansky 1997 Human platelets display high-affinity receptors for thrombopoietin. Blood 89: 1896–1904
Stefanich E, Senn T, Widmer R, Fratino C, Keller GA, Fielder PJ 1997 Metabolism of thrombopoietin (TPO) in vivo : determination of the binding dynamics for TPO in mice. Blood 89: 4063–4070
Fielder PJ, Hass P, Nagel M, Stefanich E, Widmer R, Bennett GL, Keller GA, de Sauvage FJ, Eaton D 1997 Human platelets as a model for the binding and degradation of thrombopoietin. Blood 89: 2782–2788
Rasko JEJ, Basser RL, Boyd J, Mansfield R, O'Malley CJ, Hussein S, Berndt MC, Clarke K, O'Byrne J, Sheridan WP, Grigg AP, Begley CG 1997 Multilineage mobilization of peripheral blood progenitor cells in humans following administration of PEG-rHuMGDF. Br J Haematol 97: 871–880
Cwirla SE, Balasubramanian P, Duffin DJ, Wagstrom CR, Gates CM, Singer SC, Davis AM, Tansik RL, Mattheakis LC, Boytos CM, Schatz PJ, Baccanari DP, Wrighton NC, Barrett RW, Dower WJ 1997 Peptide agonist of the thrombopoietin receptor as potent as the natural cytokine. Science 276: 1696–1699
Boytos CM, Rudolph SK, Merrell C, DePrince R, Weigl D, de Serres M, Lalonde G 1998 Pegylated thrombopoietin-mimetic peptides elevate blood platelets and ablate the chemotherapy-induced platelet nadir in mice. Blood 92: 377A
Acknowledgements
The authors thank Lorin Roskos and Chris Reiber from Amgen, Inc. (Thousand Oaks, California) for running the PEG-rHuMGDF ELISA and for performing the pharmacokinetic analysis.
Author information
Authors and Affiliations
Additional information
Supported by an American Heart Association Fellowship Grant [M.C.S.], an award from the University of Florida Children's Miracle Network Telethon, and grants from the US Public Health Service: HL-44195, DK-49317, HL-55175, AI-32299, DK-53711.
Rights and permissions
About this article
Cite this article
Sola, M., Christensen, R., Hutson, A. et al. Pharmacokinetics, Pharmacodynamics, and Safety of Administering Pegylated Recombinant Megakaryocyte Growth and Development Factor to Newborn Rhesus Monkeys. Pediatr Res 47, 208 (2000). https://doi.org/10.1203/00006450-200002000-00010
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200002000-00010
This article is cited by
-
Increasing platelets without transfusion: is it time to introduce novel thrombopoietic agents in neonatal care?
Journal of Perinatology (2010)
-
Platelet reference ranges for neonates, defined using data from over 47 000 patients in a multihospital healthcare system
Journal of Perinatology (2009)
-
Strategien zur Behandlung von Thrombozytopenien im Kindesalter
Monatsschrift Kinderheilkunde (2006)
-
Epidemiologic and Outcome Studies of Patients Who Received Platelet Transfusions in the Neonatal Intensive Care Unit
Journal of Perinatology (2001)