Abstract
Esophageal atresia (EA) is often associated with cardiovascular and other malformations that are likely neural crest derived. The present study tests the hypothesis that the heart and great vessels and the thymus and parathyroids may be abnormal in the rat model of EA as a result of disturbed neural crest development. Time-mated pregnant rats received intraperitoneally on d 8 and 9 of gestation either 2 mg/kg adriamycin or vehicle. Esophageal, heart, and thymic malformations were sought under the microscope in term fetuses. The parathyroids were histologically investigated. Control fetuses had no malformations, whereas 69 of 109 fetuses exposed to adriamycin had EA and 45 of 69 had 15 right aortic arches, nine aberrant right subclavia, eight ventricular septal defects, six narrow pulmonary outflow tracts, five tetralogies of Fallot, three double outflow right ventricles, three double aortic arches, three atrial septal defects, three right ductus arteriosus, and two truncus. The thymus was absent in 19, hypoplastic in 12, and ectopic in five out of 36 fetuses with EA in which it was studied, whereas the parathyroid glands were absent in 16, single in four, and ectopic in one of the 23 fetuses with EA in which they were studied. In conclusion, the nature of the cardiovascular, thymic, and parathyroid malformations associated with EA in rats is consistent with the hypothesis of neural crest participation in their pathogenesis. Mechanisms simultaneously disturbing foregut septation, somitic segmentation, and neural crest development should be sought to explain the combined occurrence of malformations in EA.
Similar content being viewed by others
Main
Babies born with EA and tracheoesophageal fistula often bear associated malformations that likely share their pathogenic mechanisms. Some of these defects grouped under the VATER (vertebral, anorectal, tracheoesophageal, and renal) or VACTERL (vertebral, cardiac, anorectal, tracheoesophageal, renal, and limb) acronyms (1, 2) are caused most probably by pathogenic agents acting on various organs at the same embryonic developmental stages by as yet unknown mechanisms. However, other defects commonly associated with EA are consistent with involvement of the neural crest in the pathogenesis, such as some minor facial anomalies (3, 4), some components of the CHARGE association (coloboma, heart, choanal, growth retardation, genital, ear, and esophagotracheal defects) (5, 6), or CVM (7–12). Common pathogenic pathways for both EA and neural crest anomalies are particularly likely in the few patients with DiGeorge syndrome (thymic, parathyroid, thyroid, cardiovascular, and facial defects) who also have EA (13).
The recent availability of a rodent model of EA, induced by prenatal exposure to adriamycin, in which many of the associated defects are present (14–18) provides an excellent tool for investigating the extent of the involvement of the neural crest in the pathogenesis of all these defects. The present study tests the hypotheses that the CVM observed in rats with EA induced by adriamycin are neural crest related and that the thymus and parathyroids may be abnormal in them.
METHODS
Time-mated female Sprague-Dawley rats (n = 10) weighing 200–250 g received intraperitoneally 2 mg/kg adriamycin dissolved in sterile saline (0.5 mg/mL) on d 8 and 9 of gestation (d 0 = spermatozoa in unstained vaginal smear after mating) according to our previously reported procedure (15). Control rats (n = 2) received only saline on the same days. On d 21 of gestation (term = 22nd), the fetuses were removed, killed, immersed in 10% formalin, and maintained for 7 d at 18–20°C for adequate fixation. Each animal was subsequently weighed and examined under a binocular surgical microscope for external and internal anomalies and particularly EA. The heart and great vessels were carefully examined in situ, and, after dissection, the heart was weighed and anomalies of the interatrial septum and of the atrioventricular valves were observed through the atria and recorded. Hereafter, the heart was cut transversely in an equatorial plane midway between the apex and the aortic root to examine the interventricular septum, the right outflow tract, and the subaortic portion. Heart weights expressed as percentages of the body weight were described as mean ± SD, and the differences among groups were analyzed with 1-way ANOVA and the Fisher post hoc test. The association between CVM and EA was tested with the Fisher exact test. p values < 0.05 were accepted as significant.
One control and four adriamycin litters from the former groups were selected at random, and, in addition to cardiovascular assessment, the fetuses underwent specific investigation of the development of the thymus and parathyroids: After formalin fixation, the thymus was sought by dissection under binocular surgical microscope in the anterior mediastinum, and, when found, it was considered either normal or hypoplastic when it was obviously small and sometimes reduced to minimal remnants difficult to distinguish from the surrounding structures. Subsequently, blocks composed of thick transverse slices of cervical tissues from the tragus to below the lower limit of the larynx were embedded in paraffin. Serial 3-μ sections were cut in the horizontal plane, and one of every 15 was stained with hematoxylin-eosin for histologic study of branchial pouch derivatives. Thymic remnants and parathyroid tissue were carefully searched for in a craniocaudal direction until below the lower limits of the thyroid. The observer was blinded as to the treatment of each animal, although the anatomy of the pharynx and larynx in adriamycin-treated animals allows easy identification of them.
The present study, which was previously approved by the Institutional Research Committee, was performed in accord with the regulations for animal care of the European Union (E.C. 86/L609).
RESULTS
The esophagus and trachea were normal in all control animals (two litters, 21 pups), and no malformations were found in them. Conversely, 69 (63%) of the 109 fetuses from 10 litters exposed to adriamycin had EA with tracheoesophageal fistula and other malformations [urinary in 85 fetuses (78%), duodenal atresia in 69 (63%), anal atresia in 21 (19%), genital in 13 (12%), and caudal in 14 (13%)].
In contrast with control animals, which had consistently normal cardiovascular systems, 45 of 69 fetuses with EA (65.2%) had heart malformations (n = 12), great vessel malformations (n = 19), or both (n = 14). Among the 40 treated animals without EA, four had heart defects, five had great vessel malformations, and one had both. The association between the presence of EA and a CVM was significant (Fisher, p < 0.01) because 45 out of the 69 fetuses with EA (65%) had such anomalies, whereas only 10 out of 40 without EA (25%) had them. The heart weight in EA fetuses was increased in comparison with controls and animals without EA (0.98 ± 0.21%versus 0.89 ± 0.08% and 0.84 ± 0.14% of body weight, respectively, p < 0.05). Table 1 summarizes the CVM found in adriamycin-exposed fetuses: the most frequent heart defect in those with EA was perimembranous VSD observed in 11.6% (Fig. 1A) followed by NPOT with normal infundibulum and pulmonary valve present in 8.7%, TOF in 7.2%, DORV in 4.3%, and TA with left or right arch in 2.9% (Fig. 1B). Less common were ASD (1.4%), tricuspid valve dysplasia (1.4%), or aneurysm of the sinus of Valsalva (1.4%). Anomalies of the great vessels were even more frequently seen: RAA with left ductus arteriosus (21.7%) and ARSA (13%) were most commonly observed. These anomalies created complete vascular rings around the trachea, and so did three DAA (Fig. 1C) and three RAA with RDA with or without aberrant subclavian artery. Two fetuses showed patent remnants of the embryonal intersegmental arteries, and one had a preductal CoA. Some of the same defects were also observed in smaller proportions in treated fetuses without EA (Table 1).
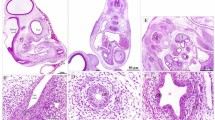
Examples of CVM observed in adriamycin-exposed rat fetuses with EA. Fixed specimens dissected under microscope. A, Perimembranous VSD (arrow) seen from the left side of the interventricular septum. B, Right TA with RAA (v, ventricle;ta, truncus;tr, trachea;l, lung;arrow, right pulmonary artery). The trachea is clearly visible along with the carotid arteries. C, DAA encircling the trachea on both sides (a, aorta;p, pulmonary artery).
The findings related to the thymus and parathyroids are shown in Table 2 and Figure 2. In the fetuses specifically investigated for this purpose, the thymus was normal in the 11 controls, and, conversely, it was absent in 19 of 36 or 52% of those with EA and grossly hypoplastic or ectopic in the remaining 17 or 47% (Table 2). The ectopic organs were found histologically in very aberrant locations (Fig. 2). Nine out of 12 animals exposed to the teratogen but without EA had very small thymuses, whereas the remaining three had normal organs.
A, Transverse section at the laryngeal level in a control rat fetus. The esophagus is visible behind the airway. On each side, thyroid lobes with the two normal parathyroids embedded in their lateral surfaces. B, Transverse section at the upper laryngeal level in an adriamycin-exposed fetus with EA. H, hyoid bone;L, larynx;V, vertebral body (notice the absence of both esophagus and pharynx). Th indicates ectopic fragment of the thymus in a high and lateral retrolaryngeal position. C, Transverse section at the midlaryngeal level in an adriamycin-exposed fetus with EA. Notice again the absence of esophagus, the vertebral body behind the larynx, the slightly asymmetrical thyroid lobes (T), and the parathyroids (Pt) located in an ectopic position behind the thyroid lobes and with the right one clearly separated from it.
In regard to the parathyroid glands, the histologic material was adequate for accurate evaluation in nine of 11 control and 30 of 48 adriamycin fetuses (seven without and 23 with EA). All controls had two evenly spherical normal glands consistently located in the lateral surface of both thyroid lobes and clearly demarcated from their parenchyma (Fig. 2A). All exposed fetuses without EA (n = 7) had normal glands on both sides except one, which had only one gland on the right side. The parathyroids were absent in 16 of 23 animals with EA (69%), four (17%) had only one gland (two right-sided and two left-sided), and another (4%) had an ectopic gland located dorsally to the thyroid and in a slightly high position (Fig. 2C). Finally, only two animals with EA (8%) had normal parathyroids.
All 23 animals with EA available for parathyroid studies had abnormal thymus (12 absent, nine grossly hypoplastic, and two ectopic), and 17 of 23 (73%) had, in addition, cardiovascular defects (seven RAA, three ARSA, three VSD, three NPOT, one DORV, one TA, one CoA, one ASD, one TOF, one RDA, one aneurysm of the sinus of Valsalva).
DISCUSSION
The adriamycin model of EA reproduces not only the anatomy of the defect (14) but also the associated gastrointestinal (14, 18), urologic (18), and skeletal (17) malformations as well as other defects such as the abnormal vagal anatomy (19) and the scarce intrinsic innervation (20, 21) previously observed in EA patients (22, 23). The mechanisms of all these malformations involve mainly abnormal tracheoesophageal and recto-urinary separation as well as abnormal segmentation of somitic derivatives, but some observations suggest that abnormal neural crest development also contributes to these pathogenic events.
Twenty to 60% of babies with EA have associated CVM such as VSD, ASD, PDA, valvular aberrations, conotruncal defects, RAA, and CoA together with aortic arch anomalies (7–12). The incidence of these defects is higher when other anomalies are present, and some particular types are more common in the context of well-recognized associations (24): VSD, ASD, and PDA are the most common anomalies in VACTERL association (25–28), whereas conotruncal or aortic arch defects are more frequent in infants with either CHARGE association (29, 30) or DiGeorge syndrome (13, 29). All these CVM anomalies except ASD and PDA are mainly neural crest derived.
Qi et al. (16) demonstrated recently that CVM were present in 18 of 24 adriamycin-exposed rat fetuses with EA and in 10 of 12 exposed animals without EA. The present study demonstrates in the same model, with different methods, comparable incidence but a wider range of malformations. In both studies, these were predominantly derived from abnormal conotruncal septation and from abnormal rearrangement of the arteries of the pharyngeal arches (perimembranous VSD, NPOT, TOF, DORV, TA, RAA, ARSA, DAA, and RDA) and, therefore, were consistent with a mechanism related to the neural crest.
The primitive human cardiac tube acquires an “S” shape on the 23rd gestational day with the atria, ventricles, conotruncus (outflow tract), and aortic sac (proximal great vessels) arranged in a row. This tube is subsequently divided into left and right atrioventricular canals, whereas the conotruncus septates to form the great vessels. Simultaneously, the six pharyngeal arterial branches emerge on each side from the aortic sac. In the fifth week, the common and internal carotid arteries (third aortic arch) appear in the mesoderm of the third pharyngeal pouch, whereas the primordia of the thymus and the lower parathyroids appear in the neighboring endoderm from where they will migrate subsequently toward their final destination. The fourth aortic branches become the definitive aortic arch, the right subclavian artery, and the proximal pulmonary arteries, whereas the upper parathyroid glands arise from the endoderm of the fourth pharyngeal pouch at the same level. Finally, the sixth aortic arch forms the ductus arteriosus (31). The neural crest is undoubtedly involved in these complex organogenetic processes, along with other tissue components, and under the control of molecular pathways that are becoming progressively better understood (32). Cells from the cardiac neural crest located between the midotic placode and the caudal limit of the third somite migrate along the third, fourth, and sixth pharyngeal arches and populate their arterial axes as well as the cardiac outflow tract where they contribute to conotruncal septation and to development of the aortic arches, thymus, parathyroids, and C-cells of the thyroid (31, 32). Experimental ablation of the entire cardiac neural crest in chick embryos results in absence of septation of the conotruncus (persistence of the TA) and in a variety of malformations involving the pharyngeal arch arteries (mainly aortic arch defects) as well as the absence of the thymus, parathyroids, and thyroid depending on the extent of the ablation. Partial ablation induces incomplete septation of the conotruncus, leading to DORV (33, 34).
In addition to the nature of the CVM, there are other clinical reasons for linking neural crest-derived anomalies with EA such as the glossoptosis and abnormal respiratory tract innervation (3) and the frequent observation of minor facial anomalies in EA patients (4). Some cases of DiGeorge syndrome have EA (13) in addition to conotruncal, facial, thymic, thyroid, and parathyroid anomalies (29). Other clinical conditions associated with EA such as the CHARGE complex (6, 30), especially when it is associated with neuroblastoma (5), and Waardenburg's syndrome (35) involve neural crest-derived components. There is also a report on a human embryo with EA and many neural crest anomalies (36).
The complete absence of thymus in most animals with EA in the present study contrasts strikingly with its consistent presence in controls and reveals the failure of the third pharyngeal pouch to form its stroma. The finding of severe hypoplasia with only minimal remnants of the organ in some animals exposed to the teratogen with and without EA and the few cases of ectopic remnants of the organ would correspond to partial failure and abnormal descent of the thymus from the pharyngeal floor.
The absence of parathyroids in most EA animals and their occasional asymmetric development or ectopia reveal also a failure of the contribution of the third pharyngeal pouch. In rodents, there are only two glands that originate at this level and that, at the end of their migration, are embedded on the lateral surface of the parenchyma of the thyroid lobes (37) where they can be identified by their particular cell arrangement and staining, which are very different from those of the thyroid. Because their location is consistent and the observation of accessory glands is exceedingly rare [Hoskins and Chandler (38) found only four suprathyroid extra glands in 94 rats], we believe that our findings based on a careful search of multiple serial sections involving the entire region are consistent.
Taking into account that conotruncal septation and pharyngeal arch development occur in the rat between the 10.5th and the 12.5th gestational days, that neural crest cell migration starts shortly before that time, that tracheoesophageal separation takes place between the 11th and the 12th days (39), and that the pharyngeal pouch derivatives migrate at the same time (40), it is understandable that prenatal exposure to the teratogen can induce simultaneously CVM, thymic, parathyroid, and esophageal anomalies. However, the mechanisms of teratogen-induced malformations are rarely understood. When they appear grouped in more or less stereotyped associations or clusters, a common pathogenic mechanism should be expected, and this seems to be the case in experimental EA.
There is increasing evidence of the participation of some phylogenetically constant homeobox genes in the regulation of the organogenesis and body organization and segmentation in humans and animals alike. Deletion of some of these genes in mice produces anomalies that involve the foregut, spine, heart, and neural crest-derived tissues, allowing speculation on the possible participation of genetic mechanisms of this sort in the pathogenesis of EA and associated anomalies. Using other teratogens, we demonstrated in our laboratory (41) that their mechanism of action could be mediated through down-regulation of transcription factors that act downstream in the cascade of organogenetic events regulated by the combinatorial code of Hox genes in charge of the transmission of the body plan. These genes, in concerted and partially overlapping action, provide the basis for somitic identity, specificity, and spatial relationships of the various organs and for overall embryonic shaping (42). Disruption of Hoxc-4 in a genetically engineered mouse causes disappearance of the esophagus over a large portion of its length with profound disorganization of its wall layers along with thoracic vertebral defects similar to those found in EA (43). EA with tracheoesophageal fistula and several of the above-mentioned malformations has been observed in mutant mice with sonic hedgehog (44), Gli2 and Gli3 protein, (45) and Nkx2.1 (46) gene deletions. Mice defective for Hoxa-3 are athymic, aparathyroid, and bear heart and great vessels defects with laryngeal and tracheal cartilaginous abnormalities (47). Hox-3 paralogous genes interact to form structures of different origins such as neural crest-derived tissues and somitic mesoderm-derived tissues and have a high overlapping and reciprocal enhancing function (48–50).
The previously available evidence that adriamycin accounts for a cluster of malformations resulting from abnormal endodermal-mesenchymal interaction (EA, duodenal and anal atresias) and disturbed somitic segmentation (costovertebral and limb anomalies) in fetal rats and the previous and present evidence that other components are caused by abnormal neural crest development (CVM, vagal anomalies, scarce intrinsic innervation, and absence or hypoplasia of the thymus and parathyroids) invite consideration of the molecular mechanisms related to changes in the expression of structural genes as the main agents in the pathogenesis of EA with its associated malformations. The adriamycin rat model is the most appropriate investigative tool for further research in this field.
Abbreviations
- EA:
-
esophageal atresia
- CVM:
-
cardiovascular malformation
- VSD:
-
ventricular septal defect
- TOF:
-
tetralogy of Fallot
- NPOT:
-
narrow pulmonary outflow tract
- DORV:
-
double outlet right ventricle
- TA:
-
truncus arteriosus
- ASD:
-
atrial septal defect
- RAA:
-
right aortic arch
- ARSA:
-
aberrant right subclavian artery
- DAA:
-
double aortic arch
- RDA:
-
right ductus arteriosus
- CoA:
-
coarctation of the aorta
- PDA:
-
persistent ductus arteriosus
References
Russell LJ, Weaver DD, Bull MJ 1981 The axial mesodermal dysplasia spectrum. Pediatrics 67: 176–182
Weaver DD, Mapstone CL, Yu PL 1986 The VATER association. Am J Dis Child 140: 225–229
Cozzi F, Myers NA, Madonna S, Drago S, Fiocca G, Piacenti S, Pierro A 1991 Esophageal atresia, choanal atresia, and dysautonomia. J Pediatr Surg 26: 548–552
Cozzi F, Myers NA, Piacenti S, Orfei P, Cozzi DA, Bonanni M, Madonna L 1993 Maturational dysautonomia and facial anomalies associated with esophageal atresia: support for neural crest involvement. J Pediatr Surg 28: 798–801
Hiyama E, Yokohama T, Ichikawa T, Miyamoto K, Matsuura Y 1990 CHARGE association with neuroblastoma. Pediatr Surg Int 5: 463–465
Kutiyanawala M, Wyse RK, Brereton RJ, Spitz L, Kiely EM, Drake D, Blake K 1992 CHARGE and esophageal atresia. J Pediatr Surg 27: 558–560
German JC, Mahour GH, Woolley MM 1976 Esophageal atresia and associated anomalies. J Pediatr Surg 11: 299–306
Greenwood RD, Rosenthal A 1976 Cardiovascular malformations associated with tracheoesophageal fistula and esophageal atresia. Pediatrics 57: 87–91
Chittmittrapap S, Spitz L, Kiely EM, Brereton RJ 1989 Oesophageal atresia and associated anomalies. Arch Dis Child 64: 364–368
Ein SH, Shandling B, Wesson D, Filler RM 1989 Esophageal atresia with distal tracheoesophageal fistula: associated anomalies and prognosis in the 1980s. J Pediatr Surg 24: 1055–1059
Mee RBB, Beasley SW, Auldist AW, Myers NA 1992 Influence of congenital heart disease on management of oesophageal atresia. Pediatr Surg Int 7: 90–93
Rokitansky A, Kolankaya A, Bichler B, Mayr J, Menardi G 1994 Analysis of 309 cases of esophageal atresia for associated congenital malformations. Am J Perinatol 11: 123–128
Palacios J, Gamallo C, Garcia M, Rodriguez JI 1993 Decrease in thyrocalcitonin-containing cells and analysis of other congenital anomalies in 11 patients with DiGeorge anomaly. Am J Med Genet 46: 641–646
Diez-Pardo JA, Qi B, Navarro C, Tovar JA 1996 A new rodent experimental model of oesophageal atresia and tracheo-oesophageal fistula: preliminary report. J Pediatr Surg 31: 498–502
Qi BQ, Diez-Pardo J, Navarro C, Tovar JA 1996 Narrowing the embryologic window of the adriamycin-induced fetal rat model of esophageal atresia and tracheo-esophageal fistula. Pediatr Surg Int 11: 444–447
Qi BQ, Merei J, Farmer P, Hasthorpe S, Myers NA, Beasley SW, Hutson JM 1997 Cardiovascular malformations in rat fetuses with oesophageal atresia and tracheo-oesophageal fistula induced by adriamycin. Pediatr Surg Int 12: 556–564
Xia H, Migliazza L, Montedonico S, Rodriguez JI, Diez-Pardo JA, Tovar JA 1999 Skeletal malformations associated with esophageal atresia: clinical and experimental studies. J Pediatr Surg 34: 1385–1392
Merei J, Hasthorpe S, Farmer P, Hutson JM 1999 Visceral anomalies in prenatally adriamycin-exposed rat fetuses: a model for the VATER association. Pediatr Surg Int 15: 11–16
Qi BQ, Merei J, Farmer P, Hasthorpe S, Myers NA, Beasley SW, Hutson JM 1997 The vagus and recurrent laryngeal nerves in the rodent experimental model of esophageal atresia. J Pediatr Surg 32: 1580–1586
Cheng W, Bishop AE, Spitz L, Polak JM 1999 Abnormal enteric nerve morphology in atretic esophagus of fetal rats with adriamycin-induced esophageal atresia. Pediatr Surg Int 15: 8–10
Qi BQ, Uemura S, Farmer P, Myers NA, Hutson JM 1999 Intrinsic innervation of the oesophagus in fetal rats with oesophageal atresia. Pediatr Surg Int 15: 2–7
Davies MRQ 1996 Anatomy of the extrinsic nerve supply of the oesophagus in oesophageal atresia of the common type. Pediatr Surg Int 11: 230–233
Hokama A, Myers NA, Kent M, Campbell PE, Chow CW 1986 Esophageal atresia with tracheo-esophageal fistula: a histopathological study. Pediatr Surg Int 1: 117–121
Ein SH, Izukawa T, Su WJ, Cook D, Stephens A, Rowe RD 1998 The influence of cardiovascular malformations on the prognosis of esophageal atresia and distal tracheoesophageal fistula. Pediatr Surg Int 4: 318–321
Muraji T, Mahour GH 1984 Surgical problems in patients with VATER-associated anomalies. J Pediatr Surg 19: 550–554
Corsello G, Maresi E, Corrao AM, Dimita U, Lo Cascio M, Cammarata M, Giuffre L 1992 VATER/VACTERL association: clinical variability and expanding phenotype including laryngeal stenosis. Am J Med Genet 44: 813–815
Iuchtman M, Brereton R, Spitz L, Kiely EM, Drake D 1992 Morbidity and mortality in 46 patients with the VACTERL association. Isr J Med Sci 28: 281–284
Huang LW, Chen MR, Lin SP, Huang FY, Ho MY, Kao HA, Hsu CH, Huung HY, Tsai TC 1995 The VATER association: analysis of forty-six cases without karyotyping. PMID 36: 30–34
Jones MC 1990 The neurocristopathies: reinterpretation based upon the mechanism of abnormal morphogenesis. Cleft Palate J 27: 136–140
Pagon RA, Graham JM, Zonana J, Yong SL 1981 Coloboma, congenital heart disease, and choanal atresia with multiple anomalies: CHARGE association. J Pediatr 99: 223–227
Larsen WJ 1997 Human Embryology, 2nd Ed. Churchill Livingstone, New York, pp 189–196
Olson EN, Srivastava E 1996 Molecular pathways controlling heart development. Science 272: 671–676
Kirby ML, Waldo KL 1990 Role of the neural crest in congenital heart disease. Circulation 82: 332–340
Kirby ML, Waldo KL 1995 Neural crest and cardiovascular patterning. Circ Res 77: 211–215
Schweitzer VG, Clack TD 1984 Waardenburg's syndrome: a case report with CT scanning and cochleovestibular evaluation. Int J Pediatr Otorhinolaryngol 7: 311–322
Schenk VWD, Geene MJ, Klein HW, Kredeit P, Stefanko S 1976 A human embryo of 28-mm crown-rump length with cerebral, esophagotracheal, and cardiovascular malformations. Anat Embryol 150: 53–62
Greene EC 1963 Anatomy of the Rat. Hafner Publishing Company, NY and London, pp 109–112
Hoskins MM, Chandler SB 1925 Accessory parathyroids in the rat. Anat Rec 30: 95–99
Possoegel AK, Diez-Pardo JA, Morales C, Navarro C, Tovar JA 1998 Embryology of esophageal atresia in the adriamycin rat model. J Pediatr Surg 33: 606–612
Kaufman MH 1992 The Atlas of Mouse Development. Academic Press Limited, London., pp 213–227, 252, 265
Losada A, Xia H, Migliazza L, Diez-Pardo JA, Santisteban P, Tovar JA 1999 Lung hypoplasia caused by nitrofen is mediated by down-regulation of the transcription factor TTF-1. Pediatr Surg Int 15: 188–191
Mark M, Rijli FM, Chambon P 1997 Homeobox genes in embryogenesis and pathogenesis. Pediatr Res 42: 421–429
Boulet AM, Capecchi MR 1996 Targeted disruption of hoxc-4 causes esophageal defects and vertebral transformations. Dev Biol 177: 232–249
Litingtung Y, Lei L, Westphal H, Chiang C 1998 Sonic hedgehog is essential to foregut development. Nat Genet 20: 58–61
Motoyama J, Liu J, Mo R, Ding Q, Post M, Hui CC 1998 Essential function of Gli2 and Gli3 in the formation of lung, trachea, and oesophagus. Nat Genet 20: 54–57
Minoo P, Su G, Drum H, Bringas P, Kimura S 1999 Defects in tracheoesophageal and lung morphogenesis in Nkx 2: 1(-/-) mouse embryos. Dev Biol 209: 60–71
Chisaka O, Capecchi MR 1991 Regionally restricted developmental defects resulting from targeted disruption of the mouse homeobox gene hox 1: 5. Nature 350: 473–479
Manley NR, Capecchi MR 1995 The role of Hoxa-3 in mouse thymus and thyroid development. Development 121: 1989–2003
Manley NR, Capecchi MR 1997 Hox group 3 paralogous genes act synergistically in the formation of somitic and neural crest-derived structures. Dev Biol 192: 274–288
Manley NR, Capecchi MR 1998 Hox group 3 paralogs regulate the development and migration of the thymus, thyroid, and parathyroid glands. Dev Biol 195: 1–15
Author information
Authors and Affiliations
Additional information
Supported in part by FIS (Fondo de Investigación Sanitaria, Ministerio de Sanidad y Consumo, Spain) grants #96/0059–01 and #96/1402.
Rights and permissions
About this article
Cite this article
Otten, C., Migliazza, L., Xia, H. et al. Neural Crest-Derived Defects in Experimental Esophageal Atresia. Pediatr Res 47, 178 (2000). https://doi.org/10.1203/00006450-200002000-00005
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-200002000-00005
This article is cited by
-
Tracheo-Esophageal Fistula (TEF) in a Newborn Following Maternal Antenatal Exposure to Olanzapine
Drug Safety - Case Reports (2017)
-
Lung hypoplasia in rats with esophageal atresia and tracheo–esophageal fistula
Pediatric Research (2012)
-
Expression of homeotic genes Hoxa3, Hoxb3, Hoxd3 and Hoxc4 is decreased in the lungs but not in the hearts of adriamycin-exposed mice
Pediatric Surgery International (2007)
-
Impact of preoperative diagnosis of congenital heart disease on the treatment of esophageal atresia
Pediatric Surgery International (2006)
-
Decrease of parafollicular thyroid C-cells in experimental esophageal atresia: further evidence of a neural crest pathogenic pathway
Pediatric Surgery International (2005)