Abstract
The postoperative intestinal dysmotility seen in intestinal atresia (IA) is usually found in association with a dilatation of the proximal intestinal segment, but the etiology of this disorder is not yet fully understood. A chick IA model was made by cutting the postumbilical midgut on d 11 in ovo. The operated chicks were euthanized 2 d after hatching. The samples were divided into two groups according to the extent of the dilatation of proximal ileal segments. Cryostat sections were processed for immunohistochemistry by the use of antisera to protein gene product 9.5, vasoactive intestinal polypeptide, substance-P, and α-smooth muscle actin and were also stained by NADPH-diaphorase. In highly dilated proximal segments, a decreased number of protein gene product 9.5-positive fibers was found in both the circular muscle and submucous layers. The number of nerve fibers positive for vasoactive intestinal polypeptide, substance-P, and NADPH-diaphorase also decreased in the circular muscle layer, particularly in the deep muscular plexus. Hypertrophy and an alteration of the staining intensities in the circular muscle layer were also revealed by α-smooth muscle actin staining. The nerve distribution of the distal segments was indistinguishable from that of the age-matched controls and the sham-operated group. Abnormalities in the intramural nerves are only found in the proximal ileal segment of the IA models. The abnormal nerve distribution of the proximal segment might thus be implicated in the postoperative dysmotility of the intestine in IA.
Similar content being viewed by others
Main
Intestinal atresia (IA) is the most common disease in the field of neonatal surgery. Jejunoileal atresia occurs more frequently than duodenal or colonic atresia(1). Anatomically, the classification proposed by Louw(2) has been well known. This classification was modified by Grosfeld et al.(3). The Grosfeld classification includes the following four types. In type I, the diseased bowel wall and mesentery are seemingly intact, but the obstructed site has an intraluminal membrane. In type II, the bowel ends are separated by a fibrous cord. In type IIIa, the blind ends are separated by a V-shaped mesenteric defect, and in type IIIb, apple peel-like appearances are observed. In type IV, multiple atresias are found, sometimes having the morphologic appearance of a string of sausages.
Recently, operative techniques and perioperative management, including nutritional therapy, have led to an improvement in the postoperative outcome of patients with IA. However, some problems still remain to be solved regarding this disease. One problem is the occurrence of postoperative enteropathy, which is characterized by the dilatation and dysmotility of the proximal intestine. When the dilated intestine proximal to the obstructive site is not adequately resected out, then enteropathy is occasionally observed(4–6). There have been several studies concerning the pathogenesis of enteropathy using either histochemical or physiologic methods(7–13). It is well documented that the blindly ended proximal bowel is dilated and hypertrophied with a deficiency of peristaltic activity, whereas the distal small bowel is unused but potentially normal. These proximal changes are supposed to be results mainly from the local ischemia. Additionally, the dilatation following the obstruction per se also elicits the deficiency of peristaltic activity. Therefore, the postoperative dysmotility partly results from diminished intraluminal pressure caused by the proximal dilatation. However, the real pathogenesis in postoperative dysmotility of IA still remains to be elucidated.
Most cases with IA have been ascribed to the results of vascular occlusion of the mesenterium during the fetal period. This proposal has been documented in several mammalian and chick models(14–18). Therefore, ischemia must influence the development of both the intestinal segments proximal and distal to the obstructive site. Simultaneously, the dilatation of the proximal intestine gradually proceeds in accordance with the completion of the obstruction. It wound thus be expected that a dilatation of the proximal intestine can also cause damage to the enteric nervous system, which thus results in postoperative enteropathy. Until recently, little information has been available regarding the abnormal development of the enteric nervous system in the dilated proximal segment. In the present study, the enteric nerves were immunohistochemically analyzed in the dilated proximal segment of a chick IA model, whose midgut was cut during the embryonic period. This report thus represents the first detailed study on the influence of distension on the distribution of the intramural neurons, including nitrergic neurons, in a chick IA model.
MATERIALS AND METHODS
Animals. We used fertilized eggs from white Leghorn hens (Kyudo Co. Ltd., Tosu, Japan). These eggs were placed in an incubator (TS15, Ashida Sangyo Co. Ltd., Okayama, Japan) equipped with an automatic control system for hourly tilting, adequate temperature (37.8°C), and humidity (80%).
Embryonic surgery. The chick embryos thus underwent an operation on the 11th day of incubation, when the so called physiologic umbilical hernia appears, as shown by Tibboel et al.(17,18). A 1-cm hole was made at the top of the shell of the egg. The amniotic membrane was then carefully opened through the allantoic cavity. Subsequently, the umbilical ring was grasped with microscopic forceps, and the umbilical wall was adequately opened. The postumbilical loop of the midgut was eviscerated and cut antimesenterically. After the loop was returned to the celomic cavity, the hole of the shell was covered with plastic adhesive. Apart from the above operated chicks, the sham-operated group (n = 15), in which the allantoic and amniotic membrane was opened and then the midgut was eviscerated and handled without cutting, was also made. Thereafter, the operated and sham-operated eggs were then returned to the incubator for further development. The procedures were all performed under an operating microscope (K-880, Konan Camera R&I Inc., Tokyo).
Sampling and tissue preparations. After hatching (term, 21 d), all chicks were then bred only until 2 d old without per os. Thereafter, the chicks were euthanized at 2 d of age under deep anesthesia with ether, because the operated chicks all tended to die from a rupture of the dilated proximal ileal wall or sepsis noticed from d 4 to 5 d after hatching. The 4-cm proximal and 4-cm distal intestinal segments to the obstructed portion were then resected from operated chicks. However, the 1-cm proximal and 1-cm distal intestinal stumps to the obstructed site were not analyzed to exclude the affect of the ischemic reactions and wound healing with cutting the bowel. The chicks of the nonoperated control group (n = 17) were grown under the same conditions that the operated and sham-operated groups of chick embryos were incubated. There were no differences in the macroscopic findings under laparotomy between the control group and the sham-operated group. Therefore, the ileum measuring 4 cm in length, from both groups, was resected at 4 cm proximal to the ileocecal junction and used as controls or as samples of the sham group.
The tissue samples were immediately flushed with 0.01 M PBS (pH 7.4) and fixed in a mixture of 4% paraformaldehyde an 0.05% picric acid in 0.05 M phosphate buffer (PB, pH 7.3) for 1 h at room temperature. They were then washed in PB containing 7% sucrose and left overnight at 4°C. After being immersed in PB containing 15% sucrose for at least 1 h, they were then cut into small blocks (about 1 cm in length) and embedded in an embedding matrix (Lipshaw, Pittsburgh). Sections of 14-µm thickness were made on the cryostat, placed onto gelatin-coated glass slides, and then air-dried for 10 min with an electric fan. The 30 sections per each small tissue block were prepared for the following analysis. The sections were histochemically evaluated between the proximal and distal samples from individual chicks by means of blind observations of three evaluators (K.M., O.N., G.R.-S.) without any information and then were compared with those of normal chicks under the same conditions.
Selected sections of each sample were stained with hematoxylin and eosin for the histologic examination.
Immunohistochemical study. Protein gene product (PGP) 9.5 staining was used as a general neuronal marker. Both the NADPH-diaphorase (NADPH-d) activity and vasoactive intestinal polypeptide (VIP) were demonstrated to examine the inhibitory neuronal elements, and substance-P (S-P) staining was performed to investigate the excitatory neurons. Further, α-smooth muscle actin (α-SMA) staining was also performed to ascertain any alterations of the muscular layers. The specificity of rabbit anti-VIP antiserum has been described(19). The specificity of the histochemical reaction for NADPH-d was also tested by omission of the enzyme substrate.
The sections were incubated with 10% normal goat serum for 30 min in a moist chamber and then were incubated overnight at 4°C with a primary polyclonal antiserum to PGP 9.5, VIP, or S-P (Table 1). The sections were then washed in PBS and incubated for 1 h at room temperature with biotinylated goat anti-rabbit IgG antiserum (Vector Lab., CA). When the sections were incubated with a monoclonal anti-α SMA antiserum, 10% normal horse serum and biotinylated horse anti-mouse antiserum (Vector Lab.) were used, respectively. They were washed in PBS and incubated for 30 min at room temperature with streptavidin-FITC (0.5 µg/mL, Vector Lab.). After washing, the sections were mounted with a mounting medium for fluorescence microscopy (Vector Lab.). The preparations were examined and photographed under a Zeiss fluorescence microscope (Axioskop 50, Germany) equipped with an epi-illumination system.
The NADPH-d activity was demonstrated according to the method of Scherer-Singler et al.(20). Cryostat sections were incubated in a solution containing 0.25 mg/mL nitroblue tetrazolium, 1 mg/mL β-NADPH, and 0.5% Triton X-100 in 0.1 M Tris-HCl buffer (pH 7.6) for 30 min at 37°C. The preparations were then examined and photographed under a light microscope.
RESULTS
A total of 196 chick embryos underwent an operation on the 11th day of incubation. Of the 196 embryos, 76 chicks hatched, and 54 (71.1%) demonstrated intestines that were completely obstructed, whereas three (3.9%) were incompletely obstructed and 19 (25.0%) showed a normal appearance. As a result, the survival rate was 38.8%, and the success rate of the embryonic operation was 29%. All the intestines in the completely obstructed chicks were interrupted with a defect of the mesenterium, and the macroscopic appearance was also similar to that of type IIIa in human intestinal atresia (Fig. 1A) (3). In the completely obstructed chicks, all the ileal segments proximal to the cutting site were prominently dilated as compared with either the distal segments or the normal ileums of the controls, and the duodenum and the jejunum were slightly dilated in comparison with those of the control chicks (Fig. 1). Individual differences in degree of the proximal ileal dilatation were also observed. The ratio of the size between the maximum diameter in proximal loops and the minimum diameter in distal loops ranged from 1.9 to 4.7 (mean ± SD, 3.14 ± 0.89). The distribution of the differences was bipolar. In our preliminary experiments, the intramural nerves among the operated chicks were found to be more apparently damaged in highly dilated intestines than in moderately dilated ones. Therefore, distended bowels were divided into the following two groups according to the extent of dilatation of the proximal segment. Group 1 (moderately distended bowels) included the chicks whose diameter of the proximal intestine was less than four times as large as that of the distal one (the ratio of the size of the diameter ≦ 1 SD). Group 2 (highly distended bowels) included the chicks whose diameter of the proximal intestine was more than four times as large as that of the distal one (the ratio of the size of the diameter > 1 SD). Out of 54 chicks whose intestines were obstructed, 39 (72.2%) and 15 (27.8%) chicks belonged to groups 1 and 2, respectively. Both the proximal and distal segments from 25 out of 39 chicks in group 1 and 14 out of 15 chicks in group 2 were histochemically analyzed.
Histology (hematoxylin and eosin staining). The ileal segments from the sham-operated chicks were histologically normal and indistinguishable from those of the controls. In the obstructed proximal ileum from operated groups 1 and 2, the intestinal villi were somewhat low and wide, and the circular muscle layer was fairly thickened as compared with those of the controls. These villious and muscular changes were more conspicuous in group 2 than group 1. In contrast, the myenteric ganglia were seemingly normal in size and in number. The distal ileum from groups 1 and 2 did not show any signs of alterations in the villious, musculature, and ganglia. These findings closely resemble Tovar's experimental findings in the chick IA model(21). However, no detailed information of the other neuronal compartment could be confirmed by this technique.
Immunohistochemistry. There were no apparent distinctions in the histochemical findings between the control group and the sham-operated group. Therefore, the following descriptions in the control group also included the findings from the sham-operated chicks.
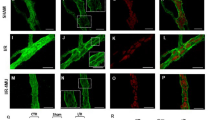
PGP 9.5 staining. A rich nerve supply was already demonstrated in the ileal wall of the 2-day-old control chicks. The nerve cell bodies positive for PGP 9.5 were localized in both the myenteric and submucous plexuses. The nerve fibers were widely distributed throughout all layers of the ileal wall, but they were mainly found in association with the circular muscle layer and submucous plexus. Sometimes, elongated axon processes from both the ganglionic plexuses were also observed to extend into the circular muscle layer. The deep muscular plexus of the circular musculature was clearly discriminated by PGP 9.5 immunostaining. A moderate supply of positive fibers was also revealed in the muscularis mucosae and the villious lamina propria. Only a few fibers were observed in the longitudinal muscle layer. Nonspecific labeling with PGP 9.5 antiserum was also found to be associated with the adipose tissue around the serous membrane (Fig. 2A).
Fluorescence micrographs of the ileum from a 2-day-old chick showing the distribution of PGP9.5-like immunoreactive nerve cells and nerve fibers (magnification 50×). Scale bar represents 50 µm. SP, submucous plexus; DP, deep muscular plexus; CM, circular musculature; MP, myenteric plexus; LM, longitudinal musculature. (A) A control ileum. Many positive cells are located in the myenteric and the submucous plexuses. Positive fibers are found in all layers of the bowel wall. (B) The proximal ileal segment from a group 1 chick. The positive nerve cells in the submucous plexus are slightly reduced, and the intramuscular nerve fibers of the circular muscle layer are less frequent. (C) The proximal ileal segment from a group 2 chick. A small number of positive cells are localized in the myenteric and the submucous plexus, whereas the lamina propia receives a scarce supply of nerve fibers. (D) The distal ileal segment from a group 2 chick. The distribution pattern of positive nerve cells and fibers is the same as that of the control.
Changes in the intramural nerves were revealed in the proximal ileal segment. In group 1, nerve cell bodies were immunolabeled by PGP 9.5 antiserum, but the axon processes could be detected less frequently. Positive nerve fibers were less numerous in each layer of the bowel wall. A most prominent feature was that the deep muscular plexus showed an equivocal immunoreactivity (Fig. 2B).
In group 2, nerve cells in both the ganglionic plexuses were faintly immunostained by PGP 9.5, and the positive nerve cells were obscure in contour. Positive nerve fibers were no longer demonstrable in the deep muscular plexus of circular musculature. The immunoreactivity in the intramuscular nerve fibers of the circular musculature was fragmental in appearance. Some fibers were somewhat intensely stained, whereas others had only a weak immunoreaction. A very low number of nerve fibers were located at the base of the lamina propia (Fig. 2C).
In contrast, nerve cell bodies and fibers in the distal ileum showed an intense immunoreactivity for PGP 9.5. The distribution pattern of the intramural nerves resembling that of the control ileum was elucidated in the distal ileal segment of both groups 1 and 2 (Fig. 2D).
Noticeable changes were found exclusively in the proximal segment of the highly distended ileal segments of the chick IA model (group 2). In contrast, mild alterations were observed in the proximal segment of group 1. No remarkable changes were found in the distal segment of groups 1 and 2. Therefore, the following descriptions were made on the findings from the IA chicks in group 2 and compared with that of controls, including the sham group.
VIP staining. A few VIP-positive nerve cells were localized in the myenteric and submucous plexuses but were prevalently found on the latter plexus in the control ileum. A considerable number of nerve fibers containing VIP were situated in the inner muscle layer of the circular musculature. The rest of the circular muscle layer received a small number of positive fibers where they were sporadically distributed. Very delicate fibers with varicosities could be observed in the subglandular connective tissue and the lamina propia (Fig. 3A).
Fluorescence micrographs of the ileum from a 2-day-old chick, showing the distribution of VIP-like immunoreactive neurons. (magnification 50×). Scale bar represents 50 µm. SP, submucous plexus; DP, deep muscular plexus; CM, circular musculature; MP, myenteric plexus; LM, longitudinal musculature. (A) A control ileum. A few number of positive cells are elucidated in the myenteric and the submucous plexuses. A considerable number of positive fibers are localized not only in the myenteric and the submucous plexus but also in the deep muscular plexus. (B) The proximal ileal segment of a group 2 chick. A reduction of VIP immunoreactive nerve cells is evident in the submucous plexus, and positive fibers are markedly decreased in the deep muscular plexus. (C) The distal ileal segment of a group 2 chick. The distribution pattern of VIP neurons is almost the same as that of the control.
In the proximal ileum of IA in group 2, nerve cell bodies positive for VIP were elucidated in the myenteric plexus but were scarce or absent in the submucous plexus. The majority of VIP-positive fibers virtually disappeared in the deep muscular plexus. The circular muscle layer was hypertrophic and innervated by a small number of positive fibers. The villious VIP fibers were either few in number or completely absent (Fig. 3B).
In the distal ileum of IA in group 2, nerve cells and fibers were somewhat weakly immunostained by VIP. Positive nerve fibers were present in the two layers of the submucous and deep muscular plexuses, and the two layers could thus be clearly distinguished (Fig. 3C).
A marked reduction of VIP-positive nerve cells and fibers was confirmed in the proximal segment of group 2, whereas only a mild reduction was found in the proximal segment of group 1 (data not shown) compared with that of controls. The VIP-nergic distribution pattern in the distal segment of group 1 was similar to that of controls, but the distribution in the distal segment of group 2 was slightly altered compared with that of the controls.
Substance-P staining. Substance-P was localized in nerve cells of the myenteric and submucous plexuses of the control chicks. A considerable number of small-sized nerve fascicules were found in the outer half of the circular muscle layer. Nerve fibers with varicosities were located in the subglandular connective tissue and villious lamina propia (Fig. 4A).
Fluorescence micrographs of the ileum from a 2-day-old chick, showing the distribution of S-P-like immunoreactive neurons. (magnification 50×). Scale bar represents 50 µm. SP, submucous plexus; DP, deep muscular plexus; CM, circular musculature; MP, myenteric plexus; LM, longitudinal musculature. (A) A control ileum. A small number of positive cells are elucidated in the myenteric and the submucous plexuses. A considerable number of positive fibers are localized not only in the myenteric and the submucous plexuses but also in the outer part of the circular muscle layer. (B) The proximal ileal segment from a group 2 chick. Note that a decreased number of positive nerve cells in the submucous plexus give rise to a marked reduction of positive fibers in the circular muscle layer. (C) The distal ileal segment from a group 2 chick. Positive nerve cells are slightly decreased in the submucous layer. No appreciable changes of the innervation pattern in the S-P-positive fibers are noticed in the circular muscle layer.
In the proximal ileum of IA in group 2, positive nerve cells were found in the myenteric plexus but not in the submucous plexus. The intramuscular nerve fascicules and the submucous nerve fibers were scarcely immunostained by S-P. No positive fibers were observed in the lamina propia, whereas occasional fibers were encountered in the subglandular plexus (Fig. 4B).
In the distal ileum of IA in group 2, nerve cells and fibers were immunostained by S-P. Positive nerve cells were present mainly in the myenteric plexus, whereas a moderate number of positive cell bodies could also be found in the submucous plexus. A moderate number of positive nerve fibers was present in the outer half of the circular musculature (Fig. 4C).
A marked reduction of S-P-positive nerve cells and fibers was also confirmed in the proximal segment of group 2, whereas only a mild reduction was found in the proximal segment of group 1 (data not shown) compared with controls. The S-P-nergic distribution pattern in the distal segment of group 1 was almost similar to that of controls, but the distribution in the distal segment of group 2 was slightly altered compared with that of controls.
NADPH-d histochemistry. NADPH-d activity was demonstrated in nerve cell bodies and processes of the ileum from the control chicks. The nucleus of positive cells was either round or oval in shape and could be seen as a nonreactive area. The perikaryon of the ganglionic cells was intensely stained, and one or more processes were observed to emanate from the cell body. Positive nerve cell bodies were localized in the myenteric and submucous plexuses. They were usually situated at the peripheral region of the myenteric plexus. Nitrergic cell bodies in the submucous plexus lay along the most outer layer of the submucosa. Nitrergic nerve fibers were densely located in the submucous plexus and the deep muscular plexus of the circular musculature. Nitrergic fibers of both plexuses were intensely stained by NADPH-d so that nitrergic cell bodies in the submucosal ganglia could not be clearly discernible. The circular muscle layer contained medium-sized nerve fascicules with NADPH-d activity (Fig. 5A).
Light micrographs of the ileum from a 2-day-old chick showing the distribution of NADPH-d activities. (magnification 100×). Scale bar represents 25 µm. SP, submucous plexus; DP, deep muscular plexus; CM, circular musculature; MP, myenteric plexus, LM: longitudinal musculature. A. A control ileum. NADPH-d activities are localized in association with the myenteric and the submucous plexuses, in which both the nerve cells and the fibers are intensely stained and small-sized nerve fascicules are observed in the circular musculature. (B) The proximal ileal segment from a group 2 chick. In contrast to the myenteric ganglia, only a few cells are positive for NADPH-d, and the intramuscular nerve fascicules also decreased in number and became smaller in size. (C) The distal ileal segment from a group 2 chick. The localization of NADPH-d resembles that of the control.
In the proximal distended ileum of IA in group 2, the myenteric nerve cells had an intense enzyme activity, but the nerve processes were much less numerous. On the other hand, most of the submucosal nerve cells were very weakly stained. A reduced number of nitrergic fibers were noticeable in the submucous plexus, the deep muscular plexus of the circular musculature, and the circular muscle layer, where almost of all the fibers had faint enzyme activities (Fig. 5B).
In the distal ileum of IA in group 2, nerve cells in the myenteric and submucous plexuses were seemingly normal compared with that of the control ileum and intensely stained by NADPH-d histochemistry. However, the nitrergic nerve fibers in the submucous and deep muscular plexuses decreased slightly in number and also showed a moderate staining intensity (Fig. 5C).
Remarkable changes in NADPH-d staining were recognized in the proximal segment of group 2 compared with that of the controls. A reduction of NADPH-d-positive nerve cells and fibers was slight in the proximal segment of group 1 (data not shown). The nitrergic innervation in the distal segment of both groups 1 and 2 was not significantly different from that of controls.
α-SMA staining. The smooth musculature of the longitudinal muscle layer, inner circular muscle layer, muscularis mucosae, and lamina propria was strongly positive for αSMA antiserum in the control ileum. The rest of the circular musculature showed a rather weak degree of immunostaining (Fig. 6A).
Fluorescence micrographs of the ileum from a 2-day-old chick, showing the distribution of α-SMA-like immunoreactivity. (magnification 25×). Scale bar represents 100 µm. MM, muscularis mucosae; IM, the inner layer of circular musculature; CM, circular musculature; LM, longitudinal musculature. (A) A control ileum. The lamina propria, the muscularis mucosae, the inner layer of circular musculature, and the longitudinal musculature are all intensely immunolabeled for α-SMA. (B) The proximal ileal segment from a group 2 chick. The circular musculature is moderately hypertrophic, whereas the thickness of the longitudinal musculature remains unchanged. (C) The distal segment from a group 2 chick. No hypertrophy of the muscle layer is recognizable.
In the proximal ileum of IA in group 2, the staining intensity of each layer became weak, and moderate hypertrophic changes were observed in the circular muscle layer, where no α-SMA-like immunoreactivity was demonstrable except for the inner circular muscle layer (Fig. 6B).
In the distal ileum of IA in group 2, the staining pattern of smooth musculature positive for α-SMA resembled that seen in the control ileal walls, although the staining intensity was slightly reduced. No hypertrophy was detected in the circular muscle layer, but the α-SMA immunoreactivity was localized only in the inner layer of the circular musculature (Fig. 6C).
The degree of the hypertrophy and staining intensity for α-SMA in the proximal segment of group 2 was higher than that of group 1 (data not shown) compared with that of the controls. The thickness and staining intensity for α-SMA in the distal segment of both group 1 and group 2 nearly resembled that of controls.
DISCUSSION
In most cases, a mesenteric vascular accident with ischemic changes during fetal life has been postulated to be the cause of IA(1). Some mammalian and avian models have been used to explore the etiology of IA(14–18). Tibboel et al.(17,18) reported that intestinal perforation or cutting at an early stage of chick embryos induces IA similar to human IA cases. The transected sites of the chick midgut are repaired and closed at least within 8-48 h after the cutting procedure(17,22). The occurrence of variant distended proximal bowels in the chick IA remains a matter for speculation. A possible explanation is that the transected midgut might be rapidly closed after operation and an early completion of obstruction might give rise to a large-sized distension of the proximal bowel. Alternatively, a delay of the closure of operated midgut might result in a smaller sized distension of the bowel. It has been shown that the intestinal development after the atresia formation closely resembles that in human IA cases(17,22), although the cutting of the midgut loop could never occur spontaneously in human IA cases. The procedure of making the intestinal atresia itself might make the influence to the intramural nerves and smooth muscle. However, the main influence owing to making this model is probably thought to be localized around the cutting site. The present study planned to investigate the intramural nerves and smooth muscle in extensive parts proximal and distal to the obstructed site. Thus, the present study was concerned with the chick IA model, which can be produced using relatively simple techniques, namely by cutting the midgut.
It is interesting to clarify whether changes in the neuronal elements of the bowel proximal to the obstructive site occur or whether the development of the intramural nerves is different between mildly and severely dilated intestines. All the enteric nerves reactive for PGP 9.5, VIP, S-P, and NADPH-d were reduced in the proximal segment, whereas no appreciable changes were demonstrable in the distal segment. Changes in the intramural nerves were conspicuous in highly dilated proximal segments (group 2). Based on these findings, it is suggested that the severe dilatation of the bowels affects the development of the enteric nervous system, particularly of the submucous plexus and the inner muscular plexus. Increasing evidence has been provided that ganglionic cells in the submucous plexus project to the axon processes into the inner circular muscle layer and thus play a significant role in intestinal motility(23,24). It seems reasonable to assume that the decrease of the neuronal elements at these locations might have caused the postoperative enteropathy in the dilated proximal intestine. VIP and nitric oxide have been postulated to be putative neurotransmitters involved in the nonadrenergic and noncholinergic relaxation of intestinal smooth muscle(25–29). Nitrergic nerves can be demonstrated by NADPH-d histochemistry(30–32). Substance-P is thought to be an excitatory transmitter both to the intestinal smooth muscle and enteric neurons(33). VIP and S-P may also play a role in the regulation of mucosal functions(34–36). The loss or reduction of these peptidergic and nitrergic nerves must contribute significantly to the pathophysiology of the severely dilated ileal segment.
Tepas et al.(7) experimentally produced IA in fetal lambs by an intrauterine disruption of the mesenteric blood supply. A histochemical evaluation using acethylcholinesterase and AT-Pase staining showed that the dilatation of the proximal segment initially induces hyperplasia of the myenteric ganglion cells, thereafter followed by the involution and lysis of the cells as the irreversible distension continues. Similar pathologic changes in myenteric ganglion cells were also found in three cases of neonates with IA, in which the antimesenteric ganglia were more severely damaged than the mesenteric ones(8). These findings contrast sharply with those of the present study, suggesting that the pathologic alterations of enteric nervous tissue initially occur in the submucosal and deep muscular plexuses. In the present experiments, the possibility that ischemia might also be implicated in nerve damage cannot be entirely ruled out. So, the ileal samples of approximately 1 cm long proximal and distal to the obstructed site were not used to rule out the influence of ischemia. Either the intramural paravascular nerve bundle or the perivascular plexus, which each is indicative of extrinsic nerves, was positive for PGP 9.5 and seemed to be normal. No topographic differences in the nerve alteration were encountered in the dilated proximal segment of the thick IA model. After the cutting procedure, most, if not all, neuronal connections must be interrupted between the interception of many enteric reflexes. The lack of neural input from the proximal segment may affect the development and maintenance of neuronal elements in the distal segment(37). In fact, a slight damage was found in peptidergic nerves containing VIP or S-P of the distal segment from group 2 chicks. When the jejunum of the fetal lambs was obstructed by a single ligature, some atrophy of the submucous ganglia was found in the proximal segment(9). It is conceivable that the mesenteric vascular disruption causes a development abnormality of neuronal elements in a manner different from that of the two IA models mentioned above.
Physiologic evidence has been provided regarding the postoperative dysmotility of bowels with IA(10–13). Cezard et al.(10) studied the postoperative duodenal function in infants with a transient enterostomy, including three cases with IA. The manometric measurement of all IA cases showed either an absence or an abnormal phase III of the migrating motor complex. It was suggested that the postoperative intestinal dysmotility is the result of a chronic change in the proximal intestine produced by either prenatal or postnatal intestinal obstruction. Doolin et al.(11) myoelectronically evaluated the postoperative motor activity of both the proximal and distal segments in lamb IA model. The spike potential activity, indicating a contraction of the circular musculature, was found to be reduced in both segments, although a reduction of neuronal elements was prevalent in the distal microintestine. On the other hand, the spike potential activity of the distal intestine in the lamb IA model was myoelectronically normal in long-term survivors(12). Takahashi et al.(13) demonstrated that the severe postoperative dysmotility in a human IA case can be improved by an additional operation, in which the plicae are re-established in the distended bowel. The most noteworthy finding is that a long-lasting distension following a prenatal intestinal obstruction may participate in the etiology of the intestinal dysmotility.
Regarding the muscular hypertrophy of the distended intestine, our present findings are in accord with those of previous reports(7–9), in which the circular musculature of the proximal intestinal segment is exclusively thickened. It has been suggested that the hypertrophy in such cases results from a prenatal intestinal obstruction. The present chick IA model provided evidence that the degree of hypertrophy in the circular muscle layer of the proximal segments are related to that of the dilatation of the proximal intestines and that the staining intensities for α-SMA decrease in the severely dilated proximal ileum of IA. However, it could not be ascertained as to whether the suppressed staining intensity for α-SMA is the result of alterations in the expression of α-SMA or because of a decrease in the α-SMA-positive muscle fibers. Previous reports have also demonstrated a reduction of the muscular ATPase activity in the dilated intestines of IA(7–9). It was suggested that the dilatation of the intestines might cause damage to the muscle layers and thus lead to the muscular functional disorder, according to the degree of the distended proximal bowel.
In conclusion, this chick IA model produced by cutting the midgut could not be referred to as an appropriate model comparable with human IA cases, except that a dilation of the proximal intestinal segment is developed. In the present study, it was not possible to determine whether the distension is the result of the intramural nervous abnormality or if an increased distension is the primary cause of the nerve damage. The intramural nervous changes was found preferentially in the proximal distended bowels, and the degree of nervous changes was parallel to that of the intestinal distension. Interestingly, the postoperative dysmotility in human IA cases has been often encountered in the highly dilated proximal intestinal segment(38). The enteric nervous and muscular changes in the chick IA model were best appreciated in sections stained by means of immunohistochemistry. It is, thus, suggested that the affected bowel segment in human IA cases had better be immunohistochemically examined to evaluate the degree of the enteric nervous changes.
Abbreviations
- IA:
-
intestinal atresia
- PB:
-
phosphate buffer
- PGP 9.5:
-
protein gene product 9.5
- VIP:
-
vasoactive intestinal polypeptide
- S-P:
-
substance-P
- NADPH-d:
-
NADPH-diaphorase
- α-SMA:
-
α-smooth muscle actin
References
Grosfeld JL 1986 Jejunoileal atresia and stenosis. In: Welch KJ, Randolph JG, Ravitch MM, O'Neill JA Jr, Rowe MI (eds) Pediatric Surgery, 4th ed. Year Book Medical Publishers Inc, Chicago, 838–848.
Louw JH 1967 Resection and end-to-end anastomosis in the management of atresia and stenosis of the small bowel. Surgery 62: 940–950.
Grosfeld JL, Ballantine TVN, Shoemaker R 1979 Operative management of intestinal atresia and stenosis based on pathologic findings. J Pediatr Surg 14: 368–375.
Nixon HH 1955 Intestinal obstruction in the newborn. Arch Dis Child 30: 13–22.
Weber TR, Vane DW, Grosfeld JL 1982 Tapering enteroplasty in infants with bowel atresia and short gut. Arch Surg 117: 684–689.
de Lorimier AA, Harrison MR 1983 Intestinal plication in the treatment of atresia. J Pediatr Surg 18: 734–737.
Tepas JJ, Wyllie RG, Shermeta DW, Inon AE, Pickard LR, Haller JA 1979 Comparison of histochemical studies of intestinal atresia in the human newborn and fetal lamb. J Pediatr Surg 14: 376–380.
Hamdy MH, Man DWK, Bain D, Kirkland IS 1986 Histochemical changes in intestinal atresia and its implications on surgical managements: a preliminary report. J Pediatr Surg 21: 17–21.
Pickard LR, Santoro S, Wyllie RG, Haller JA 1981 Histochemical studies of experimental fetal intestinal obstruction. J Pediatr Surg 16: 256–260.
Cezard JP, Cargill G, Faure C, Boige N, Mashako LMN, Munck A, Aigrain Y, Navarro J 1993 Duodenal manometry in postobstructive enteropathy in infants with a transient enterostomy. J Pediatr Surg 28: 1481–1485.
Doolin EJ, Ormsbee HS, Hill JL 1987 Motility abnormality in intestinal atresia. J Pediatr Surg 22: 320–324.
Pawlik NA, Hardy FE, Hill JL 1987 Myoelectric activity differences in acute and chronic models of lamb intestinal atresia. J Pediatr Surg 22: 1203–1206.
Takahashi A, Tomomasa T, Suzuki N, Kuroiwa M, Tabata M, Matsuyama S 1995 Gastrointestinal manometry findings in a case with dilated small bowel and disturbed transit treated successfully with bowel plication. Neurogastroenterol Mot 7: 97–100.
Louw JH, Barnard CN 1955 Congenital intestinal atresia: observations on its origin. Lancet 2: 1065–1067.
Koga Y, Hayashida Y, Ikeda K, Inokuchi K, Hashimoto N 1975 Intestinal atresia in fetal dogs produced by localized ligation of mesenteric vessels. J Pediatr Surg 10: 949–953.
Abrams JS 1968 Experimental intestinal atresia. Surgery 64: 185–191.
Tibboel D, Van Der Kamp AWM, Molenaar JC 1981 The effect of experimentally induced intestinal perforation at an early developmental stage. J Pediatr Surg 16: 1017–1020.
Tibboel D, Van Der Kamp AWM, Molenaar JC 1982 An experimental study on the effect of an intestinal perforation at various developmental stages. Z Kinderchir 37: 62–66.
Yanaihara N, Sakagami M, Sato H, Yamamoto K, Hashimoto T 1977 Immunohistochemical aspects of secretin, substance P, and VIP. Gastroenterology 72: 803–810.
Scherer-Singler U, Vincent SR, Kimura H, McGeer EG 1983 Demonstration of a unique population of neurons with NADPH-diaphorase histochemistry. J Neurosci Methods 9: 229–234.
Tovar JA, Sunol M, Lopez de Torre B, Camarero C, Torrado J 1991 Mucosal morphology in experimental intestinal atresia: studies in the chick embryo. J Pediatr Surg 26: 184–189.
Terakura H, Yoshinaga K, Sera Y, Fujimoto T 1990 Gut formation after transection of the midgut loop in the chick embryo. Okajimas Folia Anat Jpn 67: 183–194.
Gunn M 1968 Histological and histochemical observations on the myenteric and submucous plexuses of mammals. J Anat 102: 223–239.
Timmermans JP, Scheuermann DW, Stach W, Adriaensen D, De Groodt-Lasseel MHA 1990 Distinct distribution of CGRP-, enkephalin-, galanin-, neuromedin U-, neuropeptide Y-, somatostatin-, substance P-, VIP-, and serotonin-containing neurons in the two submucosal ganglionic neural networks of the porcine small intestine. Cell Tissue Res 260: 367–379.
Furness JB, Costa M 1982 Enteric inhibitory nerves and VIP. In: Said SI (ed) Vasoactive Intestinal Peptide. Raven Press, New York, 391–406.
Goyal RK, Rattan S 1980 VIP as possible neurotransmitter of non-cholinergic non-adrenergic inhibitory neurones. Nature 288: 378–379.
Bult H, Boeckxstaens GE 1990 Nitric oxide as an inhibitory non-adrenergic non-cholinergic neurotransmitter. Nature 345: 346–347.
Sanders KM, Ward SM 1992 Nitric oxide as a mediator of nonadrenergic noncholinergic neurotransmission. Am J Physiol 262: G379–G392.
Rodriguez-Membrilla A, Martinez V, Jimenez E, Gonalons E, Vergara P 1995 Is nitric oxide the final mediator regulating the migrating myoelectric complex cycle?. Am J Physiol 268: G207–G214.
Belai A, Schmidt HHHW, Hoyle CHW, Hassall CJ, Saffrey MJ, Moss J, Forstermann U, Murad F, Burnstock G 1992 Colocalization of nitric oxide synthase and NADPH-diaphorase in the myenteric plexus of the rat gut. Neurosci Lett 143: 60–64.
Young HM, Furness JB, Shuttleworth CWR, Bredt DS, Snyder SH 1992 Colocalization of nitric oxide synthase immunoreactivity and NADPH-diaphorase staining in neurons of the guinea-pig intestine. Histochemistry 97: 375–378.
Balaskas C, Saffrey MJ, Burnstock G 1995 Distribution and colocalization of NADPH-diaphorase activity, nitric oxide synthase immunoreactivity, and VIP immunoreactivity in the newly hatched chicken gut. Anat Rec 243: 10–18.
Bordin E, Alumets J, Hakanson R, Leander S, Sundler F 1981 Immunoreactive substance P in the chicken gut: distribution, development and possible functional significance. Cell Tissue Res 216: 455–469.
Carvajal SH, Mulvihill SJ 1995 Intestinal peptides and their relevance in pediatric disease. Semin Pediatr Surg 4: 9–21.
Saffrey MJ, Polak JM, Burnstock G 1982 Distribution of vasoactive intestinal polypeptide-, substance P-, enkephalin-, and neurotensin-like immunoreactive nerves in the chicken gut during development. Neuroscience 7: 279–293.
Furness JB, Costa M 1987 The Enteric Nervous System. Churchill Livingstone, London, 190–206.
Epstein ML, Poulsen KT, Thiboldeaux R 1991 Formation of ganglia in the gut of the chick embryo. J Comp Neurol 307: 189–199.
Cywes S, Rode H, Millar AJW 1994 Jejunoileal atresia and stenosis. In: Freeman NV, Burge DM, Griffiths DM, Malone PSJ (eds) Surgery of the Newborn. Churchill Livingstone, London, 117–137.
Acknowledgements
The authors thank Prof. D. Tibboel, the Department of Pediatric Surgery, Sophia Children's Hospital, Rotterdam, Netherlands, for his help and guidance in our experimental work using the chick intestinal atresia model, and Mr. Brian T. Quinn for reviewing this manuscript.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Masumoto, K., Suita, S., Nada, O. et al. Alterations of the Intramural Nervous Distributions in a Chick Intestinal Atresia Model. Pediatr Res 45, 30–37 (1999). https://doi.org/10.1203/00006450-199901000-00006
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199901000-00006
This article is cited by
-
C-Kit receptor (CD117) in the porcine urinary tract
Pediatric Surgery International (2008)
-
Cajal-like cells in the upper urinary tract: comparative study in various species
Pediatric Surgery International (2005)
-
Nervous system development in normal and atresic chick embryo intestine: an immunohistochemical study
Anatomy and Embryology (2004)