Abstract
Surfactant protein B (SP-B) is a 79-amino acid hydrophobic surfactant protein that plays a critical role in postnatal lung function. Homozygous SP-B (−/−)-deficient mice die of respiratory failure at birth, associated with severe pulmonary dysfunction and atelectasis. Heterozygous SP-B (+/−)-deficient mice have 50% less SP-B protein, proprotein, and SP-B mRNA compared with control mice and are highly susceptible to oxygen-induced lung injury. In the current study, we tested whether the susceptibility of SP-B (+/−) mice to hyperoxia was restored by intratracheal administration of exogenous SP-B. After exposure to 95% oxygen for 3 d, opening pressures were increased and maximal lung volumes were significantly decreased in SP-B (+/−) mice compared with SP-B (+/+) mice. SP-B (+/−) mice were administered purified bovine SP-B (2%) with DL-α dipalmitoyl phosphatidylcholine (DPPC) and 1-palmitoyl-2-oleoyl-sn-glycero-3-[phospho-rac-(1-glycerol)] (POPG) phospholipids or DPPC and POPG phospholipids intratracheally and exposed to 95% oxygen. SP-B–treated SP-B (+/−) mice survived longer in 95% oxygen. Although decreased lung function in SP-B (+/−) mice exposed to oxygen was not altered by administration of DPPC and POPG, administration of lipids containing 2% purified bovine SP-B restored lung function when assessed after 3 d in oxygen. Abnormalities in pulmonary function in SP-B (+/−) mice after oxygen exposure were associated with increased alveolar capillary leak, which was corrected by administration of SP-B with DPPC and POPG. Likewise, histologic abnormalities caused by oxygen-induced lung injury were improved by administration of SP-B with DPPC and POPG. Administration of phospholipids with the active SP-B peptide was sufficient to restore pulmonary function and prevent alveolar capillary leak after oxygen exposure, demonstrating the protective role of SP-B during oxygen-induced lung injury.
Similar content being viewed by others
Main
SP-B is a 79-amino acid, amphipathic polypeptide that is essential for maintaining lung function (1). Lack of SP-B in gene-targeted mice and in humans with null mutations in the SP-B gene causes lethal respiratory failure at birth (2, 3). Although SP-B (+/−) mice survive postnatally under normal vivarium conditions, lung concentrations of SP-B, proSP-B, and SP-B mRNA are reduced by 50%. Pulmonary function testing demonstrate minor abnormalities in lung compliance in room air (4). Hyperoxia causes pulmonary edema, hemorrhage, and increased alveolar-capillary leak in various animal models and induces synthesis of SP-A. SP-B, and SP-C in rodents (5). Decreased pulmonary function and increased alveolar-capillary leak are observed in SP-B (+/−) mice after exposure to 95% oxygen. Although SP-B mRNA and protein increased approximately 5-fold after exposure of SP-B (+/−) mice to 95% oxygen, SP-B and SP-B mRNA concentrations remain half that of SP-B (+/+) mice after oxygen exposure, and the animals display severe pulmonary dysfunction after 3 d in oxygen (6).
The active SP-B protein is synthesized by proteolytic processing of a larger precursor protein in type II epithelial cells (7, 8). ProSP-B and proSP-C are cotransported in vesicular compartments of type II cells (9, 10), are colocalized in the endoplasmic reticulum and multivesicular bodies of type II cells, and are processed to the active SP-B and SP-C peptides and stored in lamellar bodies. ProSP-B is required for processing proSP-C to its mature peptide and for the formation of lamellar bodies (2). Whether resistance to hyperoxic lung injury seen in SP-B (+/+) mice is related directly to the function of the active SP-B protein in the airway or to the effects of proSP-B on lipid trafficking, lamellar body formation, and proSP-C processing is unknown. Inasmuch as SP-A, SP-C, and surfactant phospholipids are also increased during oxygen exposure, it is unclear whether SP-B or proSP-B alone is sufficient for maintenance of pulmonary function during oxygen exposure. The present study was designed to test whether exogenous SP-B was sufficient to restore lung function in SP-B (+/−) mice during hyperoxia-induced lung injury.
METHODS
All procedures were approved by the Institutional Animal Care and Use Committee at the Children's Hospital Research Foundation. SP-B (+/+) and SP-B (+/−) mice were produced by mating SP-B (+/−) mice that have been kept in viral-free containment for more than 2 y in our vivarium and maintained in the FVB/N strain for more than 12 generations. SP-B–deficient mice were generated by ablation of the SP-B gene via insertion of the neomycin-resistance gene into the fourth exon of the murine gene in embryonic stem cells (D3), as previously described (2). DNA was isolated from tail samples, and the genotype was determined using PCR amplification. At 16–18 wk of age, SP-B (+/+) and SP-B (+/−) littermates were placed in a Plexiglas chamber in which the oxygen concentration was maintained at 95% at 1 atm for 72 h. A separate group of SP-B (+/−) mice was placed in 95% O2 until death. Control mice were maintained in a chamber in room air for the same time. The lipid mixture (Avanti Polar Lipids, Alabaster, AL) contained DPPC and POPG, 7:3 by weight. The lipids were diluted in chloroform, completely dried with N2, and resuspended by sonication in sterile 0.9% NaCl.
Surfactant protein purification.
SP-B was isolated and purified from C:M extracts of minced bovine lung using a combination of established chromatography methodologies (11, 12). Briefly, crude minced lung isolate was applied to a preparative silica C8 column (J.T. Baker, Inc., Phillipsburg, NY) and eluted with 47.5:47.5:5 vol/vol/vol C:M:0.1 M HCl. Fractions containing SP-B, as determined by SDS-PAGE, were pooled, reduced in volume using a stream of N2, and dialyzed against 1:1 vol/vol C:M. The SP-B pool was applied to a Sephadex LH60 column (Sigma Chemical Co., St. Louis, MO) and eluted with the same C:M:HCl solvent system as described above. Fractions containing purified SP-B were pooled and dialyzed against 1:1 C:M. SDS-PAGE was used to ascertain SP-B purity. SP-B concentration was determined by the bicinchoninic acid (BCA) total protein assay (Sigma Chemical Co.). All SDS-PAGE assays were performed using 10% acrylamide Nu-Page gels (Novex, San Diego, CA) and MOPS (3-[N-morpholino] propane sulfonic acid) running buffer supplied by the manufacturer.
Purified bovine SP-B was added to the DPPC/POPG mixture to 2%. SP-B and lipids were diluted in chloroform, completely dried, and resuspended in sterile 0.9% NaCl. Mice were anesthetized by isoflurane inhalation and received 100 mg/kg (4 mL/kg) of DPPC/POPG or SP-B/DPPC/POPG intratracheally and placed in 95% oxygen in the chamber.
Pressure-volume analysis.
Mice were injected with sodium pentobarbital (100 mg/kg i.p.) and placed in a container containing 100% oxygen to ensure complete collapse of the alveoli by oxygen absorption. The mice were killed by exsanguination, and the trachea was cannulated and connected by a syringe to a pressure sensor (Mouse Pulmonary Testing System, T.S.S. Inc., Cincinnati, OH) via a three way connector. After opening the diaphragm, lungs were inflated in 100-μL increments every 10 s to a maximum pressure of 35 cm H2O and deflated. Pressure-volume curves were generated for each animal. OP was determined as the point of the inflation curve where the curve changes from a straight line to sigmoid. V35 was determined as the volume of the lung at 35 cm H2O inflation pressure. V10, V5, and V0 were recorded as the volumes of the lung at 10, 5, and 0 cm H2O, respectively, during the deflation curve.
Permeability studies.
125I-Albumin (5 μCi of 4.45 μCi/μg, NEN Life Science Products, Boston, MA) was separated from free label with Sephadex G-50 resin and injected intraperitoneally. Two hours later, mice were killed (200 mg/kg sodium pentobarbital i.p.) and weighed. The trachea was cannulated, and the lungs were lavaged with five 1-mL aliquots of cold 0.15 M NaCl (in and out three times each) with care taken to prevent overinflation of the lungs. This procedure has been shown to recover more than 85% of alveolar radioactivity. Duplicate aliquots of the injectate and lavage fluid were counted in a gamma counter (Packard Multiprias 4, Downers Grove, IL). The total amount of radioactivity in lavage fluid was determined. The percentage of total counts in the injectate that was recovered in the lavage fluid was calculated as an estimate of albumin permeability.
Surface activity.
The surface activity of SP-B/DPPC/POPG and DPPC/POPG was assessed by captive bubble surfactometer as previously described (13), placing 3 μL of sample containing 5 μg/μL at the air-liquid interface.
Lung histology.
SP-B (+/−) mice were maintained in room air, exposed to 95% O2 for 3 d, or treated with DPPC/POPG or SP-B/DPPC/POPG before O2 exposure as described above. The mice were anesthestized with triple sedative (ketamine, acepromazine, xylazine) and exsanguinated by clipping the inferior vena cava and descending aorta. The diaphragm was slit, and the collapsed lungs were fixed by inflation to 25 cm H2O with 4% paraformaldehyde in PBS for 1 min and then immersed in cold fixative for an additional 16 h. The tissue was processed into paraffin blocks, and 5-μm-thick sections were cut and stained with hematoxylin and eosin. Multiple sections from three to four mice from each treatment group were analyzed for histopathology at the light microscopic level.
Statistical analysis.
Differences between SP-B (+/+) and SP-B (+/−) mice were assessed by two-way ANOVA. Differences between means were assessed by contrast comparisons and the Student-Newman-Keuls test.
RESULTS
Effect of 95% oxygen on lung function in SP-B (+/+) and SP-B(+/−) mice.
There were no differences in body weight among each of the four experimental groups, mean weights ranging from 32.4 ± 0.5 to 33.7 ± 0.4 g. SP-B (+/+) and SP-B (+/−) mice were exposed to 95% oxygen for 3 d (n= 8 in each group) or to room air (n= 9–10 per group), or were pretreated with a single dose of 100 mg/kg DPPC/POPG or SP-B/DPPC/POPG and exposed to 95% oxygen for 3 d (n= 8 in each group). All mice survived the 3 - d exposure to oxygen. To assess effects of the treatments on survival, separate groups of SP-B (+/−) mice were treated as above (n= 5 in each group), or given no treatment and placed in 95% oxygen. SP-B (+/−) mice treated with DPPC/POPG tolerated 95% oxygen for 5 d, compared with untreated mice, although they all died on d 6. Survival was significantly increased in mice treated with SP-B/DPPC/POPG mixture (Fig. 1).
Decreased mortality after administration of SP-B/DPPC/POPG. SP-B (+/−) mice were treated intratracheally with DPPC/POPG (lipid), SP-B/DPPC/POPG (SP-B), or given no treatment as described in “Methods.” The mice were placed in 95% oxygen and time of death was recorded. Mortality was significantly improved in the SP-B-treated group, p< 0.001.
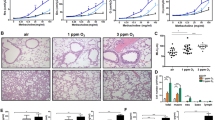
In animals exposed to room air, the OP of lungs from SP-B (+/−) mice was significantly higher than that of SP-B (+/+) mice (p< 0.01), and both V35 and V10 were significantly lower in SP-B (+/−) than in SP-B (+/+) mice (p< 0.01;Figs. 2C and 3). After lung deflation, V0 of SP-B (+/−) mice was significantly higher than that of SP-B (+/+) mice (p< 0.01), consistent with previous findings (4). Exposure to 95% oxygen for 3 d increased the OP of both SP-B (+/+) and SP-B (+/−) mice compared with those in room air (p< 0.01;Fig. 3). V35 was decreased by hyperoxia by 45% in SP-B (+/−) mice (p< 0.001) but by only 20% in SP-B (+/+) mice (p< 0.01). In SP-B (+/−) mice, V10, V5, and V0 were decreased after oxygen compared with SP-B (+/−) mice in room air (p< 0.001;Fig. 2, B and D).
Pressure-volume curves of SP-B (+/+) and SP-B (+/−) mice exposed to room air or 95% oxygen for 3 d. Each point of the pressure-volume curve represents the mean ± SE of all experimental animals. A, SP-B (+/+) mice, room air, n= 10;B, SP-B (+/+) mice, 95% oxygen, n= 8;C, SP-B (+/−) mice, room air, n= 9;D, SP-B (+/−) mice, 95% oxygen, n= 8. Lung volumes were divided by body weight of animals and are expressed as milliliters per kilogram. In room air, V35 of SP-B (+/−) mice (46.8 ± 0.69 mL/kg) was significantly lower than that of SP-B (+/+) mice (52.9 ± 1.07 mL/kg;p< 0.01). V0 of SP-B (+/−) mice was higher (25.6 ± 0.40 mL/kg) than that of SP-B (+/+) mice (20.1 ± 0.49 mL/kg;p< 0.01). Hyperoxia decreased V35 by 45% in SP-B (+/−) mice (25.9 ± 0.32 mL/kg;p< 0.001), but only 20% in SP-B (+/+) mice (42.3 ± 0.62 mL/kg;p< 0.01). V10, V5, and V0 of SP-B (+/−) mice exposed to 95% oxygen (17.9 ± 0.35, 13.1 ± 0.44, and 7.2 ± 0.50 mL/kg, respectively) dramatically decreased compared with SP-B (+/−) mice in room air (39.6 ± 0.57, 34.3 ± 0.49, and 25.6 ± 0.40 mL/kg, respectively;p< 0.001).
Effects of SP-B and hyperoxia on OP. OP was determined at the pressure at which the linear line from zero pressure became sigmoidal during the inflation cycle of the pressure-volume curve. In the room air group, OP of SP-B (+/−) mice was 20.4 ± 0.13 cm H2O, which was significantly higher than that of SP-B (+/+) mice (16.1 ± 0.26 cm H2O;p< 0.01). Hyperoxia significantly increased OP both in SP-B (+/+) mice (20.4 ± 0.12 cm H2O) and SP-B (+/−) mice (25.3 ± 0.22 cm H2O), compared with that in room air (p< 0.01;top). Pretreatment with DPPC/POPG (lipid) did not change OP of lungs from mice of either genotype, whereas OP after SP-B/DPPC/POPG (SP-B) significantly decreased OP of SP-B (+/−) mice (20.4 ± 0.21 cm H2O;p< 0.001).
Effects of SP-B replacement on lung function.
Surface properties of the 2% SP-B/DPPC/POPG and DPPC/POPG mixtures were assessed by microbubble analysis, demonstrating rapid adsorption and stability of the bubble with lower minimum surface tension in the SP-B-containing mixture (Fig. 4). Intratracheal administration of 100 mg/kg DPPC/POPG did not improve OPs or lung volumes of SP-B (+/+) and SP-B (+/−) mice after oxygen exposure (Figs. 3 and 5, A and C). In contrast, lung functions of SP-B (+/−) mice treated with SP-B/DPPC/POPG were maintained after oxygen exposure. OPs were significantly improved after treatment with SP-B/DPPC/POPG when assessed after 3 d in oxygen, being similar to those of SP-B (+/−) mice in room air (Fig. 3). Likewise, V35, V10, and V5 of SP-B/DPPC/POPG-treated SP-B (+/−) mice were restored to those of SP-B (+/−) mice in room air (Fig. 5, B and D).
Pressure-volume curves of SP-B (+/+) and SP-B (+/−) mice administered DPPC/POPG or SP-B/DPPC/POPG before oxygen exposure. Each point of the pressure-volume curve represents the mean ± SE of all experimental animals in each genotype and each treatment group. All mice were treated 3 d in 95% oxygen. A, SP-B (+/+) mice, lipid, n= 8;B, SP-B (+/+) mice, SP-B/DPPC/POPG, n= 8;C, SP-B (+/−) mice, lipid, n= 8;D, SP-B (+/−) mice, SP-B/DPPC/POPG, n= 8. Volumes were divided by body weight and expressed as milliliters per kilogram. Lipid treatment (A, C) did not restore pressure-volume curves of SP-B (+/−) mice (C) after exposure to 95% oxygen. Treatment of SP-B (+/−) mice with SP-B/DPPC/POPG (D) resulted in increased lung volumes after oxygen exposure (D). All were significantly improved compared with SP-B (+/−) mice exposed to 95% oxygen (p< 0.001) without treatment (see Fig. 2D).
Alveolar-capillary permeability.
Leakage of 125I-albumin from the systemic circulation to the lung lavage fluid was measured as an indicator of alveolar capillary permeability and was similar in SP-B (+/+) and SP-B (+/−) mice in room air (Fig. 6). In SP-B (+/+) mice, 125I-albumin permeability was increased approximately 3-fold after exposure to 95% oxygen for 3 d. In contrast, permeability was markedly increased (20-fold) during oxygen exposure in SP-B (+/−) mice. Although pretreatment with DPPC/POPG did not protect SP-B (+/−) mice during oxygen exposure, lung permeability of SP-B (+/−) mice was maintained at low levels by pretreatment with SP-B/DPPC/POPG (Fig. 6).
Lung permeability of SP-B (+/+) and SP-B (+/−) mice. Lung permeability was assessed in SP-B (+/−) and SP-B (+/+) mice in room air (top left) or after 95% oxygen (top right and bottom) for 3 d by injecting 125I-albumin i.p. 2 h before killing. Permeability was calculated as the percent of 125I-albumin recovered in bronchoalveolar lavage (BAL) fluid to total injectate. Oxygen markedly increased lung permeability in SP-B (+/−) mice (*p> 0.001 compared with room air). DPPC/POPG did not significantly improve lung permeability in oxygen-exposed SP-B (+/−) or SP-B (+/+) mice. Administration of SP-B/DPPC/POPG to SP-B (+/−) mice reduced lung permeability to that seen in room air and significantly reduced albumin leak when compared with that in oxygen-exposed SP-B (+/−) mice without pretreatment (p< 0.001).
Lung histology.
After 3 d in 95% oxygen, diffuse alveolar and capillary damage, characterized by alveolar capillary congestion, edema, microhemorrhages, and inflammation consisting of neutrophils and macrophages, was observed in the lungs of oxygen-treated SP-B (+/−) mice (Fig. 7). After 3 d in oxygen, the histologic appearance of the lungs from both SP-B/DPPC/POPG- and DPPC/POPG-treated SP-B (+/−) mice was improved compared with untreated oxygen-exposed mice (Fig. 7). Although treatment with DPPC/POPG improved lung histology, increased numbers of macrophages and vascular congestion persisted. The histologic appearance of the lungs from SP-B/DPPC/POPG-treated mice was indistinguishable from mice maintained in room air.
Lung histology after exposure to 95% oxygen. SP-B (+/−) mice were maintained in room air (A), in 95% O2 (B), or treated with DPPC/POPG (C) or SP-B/DPPC/POPG (D), and then exposed to 95% oxygen for 3 d, as described in “Methods.” After 3 d, lungs were fixed by inflation to 25 cm H2O, sectioned, and stained with hematoxylin and eosin. SP-B (+/−) mice maintained in room air (A) exhibited normal lung histology with regularly shaped alveoli. After exposure to 95% oxygen (B), diffuse alveolar and vascular damage was observed, characterized by alveolar capillary congestion, microhemorrhages, and inflammation consisting primarily of neutrophils (arrowheads). Pretreatment with DPPC/POPG (C) improved lung histology in SP-B (+/−) mice exposed to 95% oxygen, but increased numbers of macrophages (arrowheads) and congestion of small arterials and venules (arrows) were observed. Pretreatment with SP-B/DPPC/POPG (D) improved lung histology, making the sample virtually indistinguishable from animals maintained in room air (A). Photomicrographs are representative of at least three animals per group. Scale bar = 50 μm.
DISCUSSION
Oxygen toxicity often accompanies therapy for respiratory disorders and is associated with cellular injury to the lung parenchyma, activation of inflammatory cells, and alveolar-capillary leak. Reduction of surface tension at the air-liquid interface requires the rapid generation of a monolayer or multilayer of phospholipids that is thought to be disrupted during oxygen exposure, at least in part by increased alveolar-capillary leak, inactivation of surfactant by serum and cellular proteins, and oxidation of surfactant lipids and/or proteins. Spreading and stability of surfactant phospholipids are dependent on the actions of surfactant proteins. The findings that SP-B (+/−) mice have modest abnormalities in lung function under vivarium conditions (4) and that SP-B (+/−) mice are more susceptible to oxygen injury and alveolar-capillary leak after exposure to 95% oxygen (6), support the importance of SP-B in lung function in vivo. The finding that intratracheal administration of surfactant phospholipids (DPPG/POPG) provided only minimal protection of the SP-B (+/−) mice from oxygen injury, whereas addition of SP-B to the lipid mixture substantially corrected mortality, alveolar capillary leak, and oxygen-induced abnormalities in pulmonary function, demonstrates a primary role for the mature 79-amino acid SP-B protein in protection of the lung from hyperoxic injury.
SP-B (+/−) mice are deficient in both SP-B and proSP-B (2). Therefore, the finding that the active SP-B peptide protected the lung from oxygen injury suggests that maintenance of surfactant activity per se, rather than intracellular functions of proSP-B, was an important component of the protective response. ProSP-B and proSP-C are trafficked together through the synthetic pathways and processed to the active peptides during transport from multivesicular bodies to lamellar bodies. ProSP-B is required for formation of lamellar bodies and for processing of proSP-C (2, 14). Therefore, the present findings focus attention on the role of the active SP-B peptide in protection of the lung during hyperoxia.
Lack of SP-B in SP-B (−/−) mice and in human infants with genetic defects in the SP-B gene markedly disrupts intracellular and extracellular processing of both surfactant proteins and lipids (2, 3). SP-B (−/−) mice lack lamellar bodies and tubular myelin and fail to process proSP-C to the active SP-C peptide (2). Alveolar spaces fill with SP-A and proSP-C (2, 14). Although proSP-B and SP-B are reduced by 50%, metabolic and structural abnormalities in pulmonary surfactant are not observed in the SP-B (+/−) mice under normal conditions (4). Differences in lung function in the SP-B (+/−) mice seen in room air, e.g. increased OP, decreased lung volumes, and increased residual volumes , are consistent with a modest reduction in surfactant function, likely related to the 50% reduction in SP-B. Previous studies from this laboratory demonstrated no changes in phospholipid composition or pool sizes in the SP-B (+/−) mice (4). Thus, the susceptibility of SP-B (+/−) mice to oxygen response is not likely to be caused by changes in lung phospholipid concentrations. Furthermore, the addition of phospholipids (100 mg/kg) in large excess of the endogenous pool of phospholipids in the mouse lung, approximately 25 mg/kg (15), was not sufficient to prevent death from oxygen injury.
Exposure of wild-type SP-B (+/+) mice to 95% oxygen for 3 d was associated with a modest decrease in pulmonary function, associated with increased OP and decreased lung volume, findings consistent with oxygen-induced changes in surfactant function (6). In SP-B (+/+) mice, oxygen caused minor changes in lung histology and a small change in alveolar capillary leak after 3 d. In contrast, pulmonary function in the SP-B (+/−) mice was markedly impaired by 95% oxygen and was associated with decreased lung volumes and increased OPs.
Although DPPC/POPG (100 mg/kg) represents a marked expansion of lung DPPC pools, the lipids alone were not sufficient for protection during oxygen injury. The marked improvement in lung function in the SP-B (+/−) mice treated with SP-B supports the concept that maintenance of surfactant activity per se represents an important factor in the response. SP-B/DPPC/POPG was highly active, as assessed by captive bubble analysis, rapidly forming a stable monolayer and maintaining stabilization during expansion and compression, consistent with the known properties of SP-B-lipid mixtures previously studied (16, 17). All aspects of the pressure-volume curve abnormalities seen in the oxygen-exposed SP-B (+/−) mice were prevented by intratracheal administration of SP-B/DPPC/POPG, including maintenance of low OPs and lung volumes. These findings suggest a direct effect of SP-B/DPPC/POPG on surface activity in the lung. The finding that alveolar capillary leak was also decreased supports the concept that surfactant activity is an important component in the pathogenesis of lung injury during hyperoxia.
Although the present findings support a mechanism of protection dependent on the maintenance of surfactant properties by SP-B during the time of oxygen exposure, it remains possible that the presence of exogenous SP-B and/or lipids protects the lung during the early phases of oxygen-induced injury, allowing lung cells to adapt, via various pathways, by the induction of synthesis of other protective molecules. For example, SP-A, SP-B, and SP-C (5), antioxidant enzymes (18), and many other proteins are induced by hyperoxia. Because the half-life of SP-B in the adult mouse is approximately 14 h (15), it is likely that most of the SP-B administered has been cleared from the airspaces during the 3 d in oxygen. Thus, it is unclear whether SP-B maintains surface tension–lowering properties of surfactant in the alveoli or serves to enhance pulmonary cell adaptation during the early phases of oxygen-induced injury.
The present findings support previous observations in various animal models that demonstrate that whole surfactant containing SP-A, SP-B, SP-C, and lipids, as well as surfactant extracts containing SP-B, SP-C, and lipids, provide protection against oxygen-induced lung injury (19). The finding that SP-B plays a critical role in protection from oxygen-induced lung injury provides a rationale for development of therapeutic strategies to enhance SP-B content, whether through regulation of endogenous pools or by administration of exogenous SP-B. Pathologic conditions such as prematurity, injury, and inflammation diminish SP-B concentrations in the alveolus, rendering patients susceptible to subsequent oxygen-induced injury during treatment and recovery.
Abbreviations
- SP-B:
-
surfactant protein B
- SP-C:
-
surfactant protein C
- SP-A:
-
surfactant protein A
- ProSP:
-
precursor molecule for surfactant protein
- DPPC:
-
DL-α dipalmitoyl phosphatidylcholine
- POPG:
-
1-palmitoyl-2-oleoyl-sn-glycero-3-[phospho-rac-(1-glycerol)]
- C:M:
-
chloroform:methanol ratio
- OP:
-
opening pressure
- V35:
-
maximal volume on inflation of lungs to 35 cm H2O
- V10:
-
volume of the lung at 10 cm H2O during the deflation curve
- V5:
-
volume of the lung at 5 cm H2O during the deflation curve
- V0:
-
volume of the lung at 0 cm H2O during the deflation curve
References
Whitsett JA, Horowitz AD 1998 Surfactant and surfactant proteins. In: Elias JA, Fishman JA, Grippi MA, Kaiser LR, Senior RM (eds) Fishman's Pulmonary Diseases and Disorders, 3rd Ed. McGraw-Hill, New York, pp 119–127
Clark JC, Wert SE, Bachurski CJ, Stahlman MT, Stripp BR, Weaver TE, Whitsett JA 1995 Targeted disruption of the surfactant protein B gene disrupts surfactant homeostasis, causing respiratory failure in newborn mice. Proc Natl Acad Sci USA 92: 7794–7798
Nogee LM, deMello DE, Dehner LP, Colten HR 1993 Brief report: deficiency of pulmonary surfactant protein B in congenital alveolar proteinosis. N Engl J Med 328: 406–410
Clark JC, Weaver TE, Iwamoto HS, Ikegami M, Jobe AH, Hull WM, Whitsett JA 1997 Decreased lung compliance and air trapping in heterozygous SP-B deficient mice. Am J Respir Cell Mol Biol 16: 46–52
Nogee LM, Wispé JR, Clark J, Weaver TE, Whitsett JA 1991 Increased expression of pulmonary surfactant proteins in oxygen-exposed rats. Am J Respir Cell Mol Biol 4: 102–107
Tokieda K, Iwamoto HS, Bachurski C, Wert SE, Hull WM, Ikeda K, Whitsett JA 1999 SP-B deficient mice are susceptible to hyperoxic lung injury. Am J Respir Cell Mol Biol 21: 463–472
Glasser SW, Korfhagen TR, Weaver T, Pilot-Matias T, Fox JL, Whitsett JA 1987 cDNA and deduced amino acid sequence of human pulmonary surfactant-associated proteolipid SPL(Phe). Proc Natl Acad Sci USA 84: 4007–4011
Weaver TE, Whitsett JA 1989 Processing of hydrophobic pulmonary surfactant protein B in rat type II cells. Am J Physiol 257: L100–L108
Voorhout WF, Veenendaal T, Haagsman HP, Weaver TE, Whitsett JA, VanGolde LMG, Geuze HJ 1992 Intracellular processing of pulmonary surfactant protein B in an endosomal/lysosomal compartment. Am J Physiol 263: L479–L486
Voorhout WF, Weaver TE, Haagsman HP, Geuze HJ, van Golde LMJ 1993 Biosynthetic routing of pulmonary surfactant proteins in alveolar type II cells. Microsc Res Tech 26: 366–373
Wang Z, Gurel O, Baatz JE, Notter RH 1996 Acylation of pulmonary surfactant protein-C is required for its optimal surface active interactions with phospholipids. J Biol Chem 271: 19104–19109
Baatz JE, Elledge BA, Whitsett JA 1990 Surfactant protein SP-B induces ordering at the surface of model membrane bilayers. Biochemistry 29: 6714–6720
Schoel WM, SchŸrch S, Goerke J 1994 The captive bubble method for the evaluation of pulmonary surfactant: surface tension, area, and volume calculations. Biochim Biophys Acta 1200: 281–290
Vorbroker DK, Profitt SA, Nogee LM, Whitsett JA 1995 Aberrant processing of surfactant protein C (SP-C) in hereditary SP-B deficiency. Am J Physiol 268: L647–L656
Ikegami M, Jobe AH, Reed JAH, Whitsett JA 1997 Surfactant metabolic consequences of overexpression of GM-CSF in type II cells of GM-CSF deficient mice. Am J Physiol 273: L709–L714
Revak SD, Merritt TA, Degryse E, Stefani L, Courtney M, Hallman M, Cochrane CG 1988 Use of human surfactant low molecular weight apoproteins in the reconstitution of surfactant biologic activity. J Clin Invest 81: 826–833
Sarin VK, Gupta S, Leung TK, Taylor V, Ohning BL, Whitsett JA, Fox JL 1990 Biophysical and biological activity of a synthetic 8. Proc Natl Acad Sci USA 87: 2633–2637
Wong HR, Wispe JR 1997 The stress response and the lung. Am J Physiol 273: L1–L9
Loewen GM, Holm BA, Milanowski L, Wild LM, Notter RH, Matalon S 1989 Alveolar hyperoxic injury in rabbits receiving exogenous surfactant. J Appl Physiol 66: 1087–1092
Acknowledgements
The authors thank Paula Blair for tissue processing and sectioning, and Ann Maher for manuscript preparation.
Author information
Authors and Affiliations
Additional information
Supported by NIH grants HL56387 and HL38859 and the Cystic Fibrosis Foundation.Present address (K.T.): Department of Pediatrics, Keio University, Tokyo, Japan.
Rights and permissions
About this article
Cite this article
Tokieda, K., Ikegami, M., Wert, S. et al. Surfactant Protein B Corrects Oxygen-Induced Pulmonary Dysfunction in Heterozygous Surfactant Protein B–Deficient Mice. Pediatr Res 46, 708 (1999). https://doi.org/10.1203/00006450-199912000-00014
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199912000-00014
This article is cited by
-
Lung surfactant proteins as potential targets of prallethrin: An in silico approach
Toxicology and Environmental Health Sciences (2022)
-
Pilot trial of late booster doses of surfactant for ventilated premature infants
Journal of Perinatology (2011)
-
Surfactant proteins SP-B and SP-C and their precursors in bronchoalveolar lavages from children with acute and chronic inflammatory airway disease
BMC Pulmonary Medicine (2008)
-
Interstitial lung disease in children – genetic background and associated phenotypes
Respiratory Research (2005)