Abstract
We studied 133 California phenylketonuria (PKU) patients and one obligate heterozygote to delineate the molecular basis of PKU in a population with greater ethnic diversity than in previous studies, and to determine whether a correlation exists between genotype and clinical phenotype, with the latter defined by both the diagnostic pretreatment blood phenylalanine (PHE) level and cognitive (IQ) test scores. To determine PAH genotypes, we used PCR-mediated amplification, denaturing gradient gel electrophoresis, and direct sequencing on dried whole blood samples. Where possible, mutation severity was defined according to predicted in vitro PAH enzyme activity estimated by using Cos cell expression analysis for a given mutation. We then asked whether mutation severity, as defined by such expression analysis, correlated with pretreatment PHE levels or with IQ test results. A mutation was identified in 236 (88%) of 267 mutant alleles. Seventeen new mutant alleles were found; A47E, T81P, I102T, E182G, T328D, Y343P, K371R, Y387H, A389E, E422K, IVS9nt5, IVS11nt20, delS70, del364–368/del198–220, delF299, delT323, and −1C/T. In striking contrast to a number of studies in other populations, in this study, based on predicted PAH activity, we observed no correlation between mutation severity and pretreatment PHE levels. There was also no correlation between genotype and IQ. We conclude that in samples collected from an ethnically heterogeneous population, there is no correlation of mutation severity with either pretreatment PHE levels or IQ measurement in treated patients. We caution that genetic counseling in PKU should incorporate the notion that prognosis may not be predicted with precision based on mutation analysis in a given patient.
Similar content being viewed by others
Main
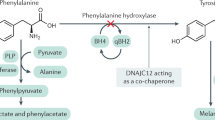
PKU is an autosomal recessive inborn error of metabolism with an incidence of 1/10,000 in Caucasians of northern European ancestry (1). The incidence among different ethnic groups varies, PKU being relatively common in Turkey and Scotland (incidences of 1/2, 500 and 1/5,000, respectively) and rare in Japan and Finland (approximate incidences between 1/100,000 and 1/200,000) (1). The disorder is caused by a mutation in the gene coding for phenylalanine hydroxylase (PAH, EC 1.14.16.1) (2). The human PAH gene is located on chromosome 12q24.1. The gene spans approximately 90 kb and contains 13 exons (3). Hyperphenylalaninemia [blood PHE level >20 μmol/L (2 mg/dL)] can also rarely be caused by defects in the synthesis or processing of tetrahydrobiopterin (BH4), an essential co-factor for PAH (1). To date, 328 mutations in the PAH gene have been reported (4), resulting in phenotypes ranging from non-PKU mild hyperphenylalaninemia (serum PHE levels <600 μmol/L = 10 mg/dL) to classic PKU (serum PHE levels >1200 μmol/L = 20 mg/dL) (5). When it has been measured, liver PAH activity is relatively high in milder forms of the disorder, whereas activity is low, and often zero, in the more severe classic form (6, 7). PKU is treated by selective restriction of phenylalanine intake (and tyrosine supplementation), while providing enough additional protein, vitamins, and minerals to support normal growth (8, 9). Mental retardation can result if diagnosis is delayed or dietary control is poor, making newborn screening the optimal diagnostic modality in the detection of hyperphenylalaninemia (1).
A number of studies have suggested a correlation between the measurable biochemical phenotype (serum PHE levels or dietary PHE tolerance) and enzyme activity of the mutant PAH gene in a Cos cell expression system (10–14). Such an expression system can indirectly lead to the definition of mutations as severe or mild, with severe mutations defined as having little or no activity in the Cos cell expression system. It was observed that such severe mutations result in a classic PKU phenotype (based on PHE levels) when present in a homozygous state, or if combined with an allele of similarly low activity in a heterozygous state. On the other hand, mild mutations (higher enzyme activity in Cos cell expression analysis) seemed to result in a less severe phenotype (10–14). Correlations between expression levels and biochemical phenotype have been reported in homogeneous European populations (10, 11), and similar correlations were observed in the population of a single state in the Southeastern United States (12). PHE tolerance (ability to eliminate a test dose of L-phenylalanine) also correlated with in vitro expression levels (13).
However, contrasting results have been observed when actual genotypes (as well as expression levels) were compared with phenotypes (15–20). Ramus et al. found major differences in IQ in several Australian patients with the same genotype, both within families and between unrelated subjects (15). Although intrafamilial phenotypic heterogeneity has been attributed to the presence of different mutant PAH alleles in some families (21, 22), different clinical phenotypes were found in three Californian siblings with the same genotype and similar PHE loading studies (16), as well as in a study of 11 Romanian PKU families (17). In a study of 106 families in a heterogeneous Belgian population, no significant correlation was found between predicted PAH activity and PHE tolerance (18). In one analysis of untreated PKU patients with mental retardation, 17% carried a mild mutation (Y414C), which can be associated with either moderate or mild PKU (19). In a related study, R261Q, predicted to be a mild mutation based on expression analysis and reported to result in a mild phenotype, can also result in severe classic PKU (20). The aggregate data suggest that whereas tentative correlations between genotype and clinical phenotype may be useful, this relationship is not straightforward, and predictive counseling must be cautionary.
To define more clearly and better understand the relationship between actual mutations and biochemical and clinical phenotype in PKU patients, we studied 134 patients in a heterogeneous California population. We determined the molecular basis of PKU in this population and compared the predicted PAH activity derived from previously reported expression analyses, the biochemical phenotype as defined as pretreatment PHE levels, and the clinical phenotype as indicated by IQ testing. This approach allowed us to test postulated relationships between genotype and phenotype, so as to provide the framework for a more accurate approximation of ultimate prognosis and optimal dietary therapy for the individual with PKU.
METHODS
Patients.
The study group consisted of 133 PKU patients and one hyperphenylalaninemic parent of a PKU patient. Ninety-three patients were initially diagnosed as having PAH deficiency through California's Newborn Screening Program and were being treated through the Divisions of Medical Genetics, Departments of Pediatrics, at Children's Hospital Los Angeles (n= 81), the University of California, San Francisco (n= 10), and Children's Hospital Medical Center, Oakland (n= 2). Forty others were diagnosed based on phenotype before universal screening was begun in 1966. In California, all patients with hyperphenylalaninemia are tested for the presence of biopterin defects. Informed consent was obtained from each patient or parent for inclusion in this study. All patients had their blood PHE and tyrosine levels, growth, development, and dietary intake monitored regularly. The ethnicity of the patients and their parents was ascertained during routine clinic visits. The study was approved by the Institutional Review Board/U.C.S.F. Committee on Human Research.
PAH mutation analysis.
Dried blood spots were used directly as a source of genomic DNA for the PCR. Either whole-blood samples were spotted onto filter papers before PCR amplification, or genomic DNA was extracted from leukocytes isolated from whole-blood samples before PCR amplification. A two-step amplification procedure was performed with three different oligonucleotide primers (23). Briefly, a first PCR reaction mixture (a 1–2 mm2 piece of the Guthrie card containing the dried whole blood or 0.5 μg of genomic DNA, 200 μmol/L deoxynucleotide triphosphates, 1.7 μmol/L Mg++, and 0.3 μmol/L of each primer in a volume of 10 μL) was denatured for 15 min at 97°C in a Perkin-Elmer (Norwalk, CT) model 9600 thermocycler. Taq DNA polymerase (0.2 U) in a volume of 10 μL was added at 85°C, and the PCR reaction proceeded for 20 cycles consisting of 1 min at 55°C, 1 min at 72°C, and 10 s at 94°C. A second PCR reaction was then performed to incorporate a GC clamp for subsequent analysis by denaturing gradient gel electrophoresis. In this reaction, 0.3 U of Taq DNA polymerase and 15 μmol/L of each GC-clamped primer and its opposite primer in a volume of 30 μL were added to the reaction tubes at 85°C, and amplification proceeded for another 40 cycles consisting of 10 s at 55°C, 20 s at 72°C, and 10 s at 94°C. Ten to 20 μL of the GC-clamped PCR product was loaded onto a 6% polyacrylamide gel containing a gradient of urea and formamide ranging from 20% to 80%. For sequencing, the PCR products were first purified from an agarose gel. For double-stranded DNA sequencing, the Taq Dye Terminator sequencing kit (Applied Biosystems, Foster City, CA) was used, and the sequencing gels were analyzed on an Applied Biosystems (Foster City, CA) model 377 automated sequencing machine.
Clinical phenotype.
The clinical variables examined in this study were: highest pretreatment PHE level in the neonatal period, PHE levels detected at the time of diagnosis in late- diagnosed patients who had not had newborn screening, IQ at 5–8 y, and latest IQ. Neonatal PHE levels were collected between day of life one and 14 d after birth. Each patient had several blood draws during this period, and the highest PHE value was used in the analysis. PHE levels in late-diagnosed patients (n= 40) were collected at ages 3 mo to 27 y, with the majority (72%) obtained before age 3 y, and all except one obtained before age 10 y. Pretreatment blood PHE levels were determined by ion-exchange chromatography on a Beckman model 6300 chromatograph (Beckman Instruments, Inc., Palo Alto, CA) using lithium buffers (24). Childhood IQ testing in patients detected by newborn screening was performed at ages 5–8 y, using Stanford-Binet IV testing (n= 70). The latest (more recent) IQ levels were obtained at ages 10–22 y in patients who were diagnosed by newborn screening, using the Wechsler Intelligence Scale for Children–Revised (WISC-R) and the Wechsler Scale for Adults–Revised (WAIS-R). Latest (more recent) IQ values in patients diagnosed on bases other than newborn screening were obtained in 34 patients, primarily by WAIS-R testing. IQ data on family members (unless they were also PKU patients, n= 3) were not available.
The frequency of specific mutant PAH alleles was compared in patients with childhood and latest IQ testing, with IQ being subdivided into three categories; <90, 90–109, and ≥ 110. (These IQ categories were chosen after preliminary analysis of the data showed that >95% of patients in the neonatally diagnosed cohort had IQ levels between 80 and 120.) Major alleles were defined as being present in >4% of a given population, minor alleles in 2–4%, and rare alleles in <2%. Comparisons were also performed, excluding patients with a history of poor control. The converse comparisons, i.e., excluding patients without a history of poor control, were also performed. Poor control was defined as the observation that >50% of a patient's measured blood PHE levels were >720 μmol/L (12 mg/L). Predicted PAH activity was defined and calculated using available activity levels from in vitro mutant PAH expression studies in Cos cells (4, 10, 12, 25). The predicted PAH activity was compared with IQ at age 5–8 y and with latest IQ in all patients whose Cos cell expression data were available for both mutant alleles. As in the analysis of frequency of mutant PAH alleles in IQ categories, comparisons were also made excluding patients with a history of poor control and excluding well-controlled patients.
Statistical analysis.
The predicted PAH activity of a given genotype was defined as the average of the activity (as a percentage of normal control levels) of the individual's two alleles. The PAH activity was calculated only when expression data for both alleles were known (10). Least squares regression analysis was used to examine the relationship between the level of PAH activity predicted from the genotype and the inverse of pretreatment serum PHE levels and subjects' IQ levels. The Pearson product-moment correlation coefficient and testing for independence at the 5% level of significance was determined by standard statistical methods. The power of the linear regression analysis was calculated by standard statistical methods. Univariate analysis was used to compare PAH activity predicted from the genotype, classified as mild, moderate, or severe, based upon predicted or measured enzymatic activity, and IQ. The Fischer exact test and the χ2 test were used to compare frequency of specific PAH mutant alleles to IQ, with p< 0.025 being regarded as statistically significant. The t test was used to compare mean IQ levels between neonatally diagnosed and late-diagnosed groups.
Calculation of PAH locus homozygosity (j).
PAH locus homozygosity was calculated according to the method of Guldberg et al. (2), where j =∑·xi2, with xi being the frequency of the ith allele. Uncharacterized alleles were assigned a frequency of 1/N, where N equaled the total number of chromosomes studied in a given population (2).
RESULTS
Identification of genotypes.
A mutation was identified in 236 (88%) of a total of 267 alleles (Table 1). In screening the California PKU population, 17 new mutations were detected (boldface, Table 1). In mutation analysis of 134 Californian PKU patients, we identified both mutant alleles in 106 subjects (80%). In 20 patients (15%), we identified only one allele. No mutations were detected in seven patients (5%). One new mutant allele was detected in a hyperphenylalaninemic mother of a child with PKU. A mutation was identified in 182 (92%) of a total of 197 alleles in patients with European (non-Armenian, non-Hispanic) heritage, 42 (75%) of 56 alleles in Hispanic patients, and 12 (86%) of 14 alleles in Armenian patients.
Genotypes in population subsets.
Patients were subdivided into European (non-Armenian, non-Hispanic) [hereafter simply referred to as “European(s)”], Hispanic, and Armenian groups, according to interviews with patients regarding ancestry. Alleles were classified as major (present in >4% of a population), minor (present in 2–4%), or rare (present in <2%). Major alleles present in the European population are R408W, IVS12nt1, IVS10nt546, I65T, and P281L. R408W and IVS10nt546 are major alleles in both European and Hispanic populations, but are present in a higher percentage in Europeans. A major mutation in Europeans, I65T, was not present in the Hispanic population. IVS12nt1 was a rare mutation in the Hispanic population, although it is present in nearly 10% of European PKU patients. Minor alleles present in the European population are as follows: F39L, L48S, F299C, L48S, R252W, L348V, and Y414C. Of these alleles, L48S, R252W, and L348V are rare alleles in the Hispanic population, whereas F39L and F299C were not detected in the Hispanic population. Nine of the rare mutations detected in the European population (T328D, Y343P, A389E, E422K, IVS9nt5, IVS11nt20, delS70, delF299, and −1C/T) are newly described alleles. The rare and newly detected mutant allele, IVS11nt20, was present in both the European and Hispanic populations (Table 1).
Major alleles present in the Hispanic population are IVS1nt5, IVS10nt546, R261Q, E280K, and R408W. Of these, IVS1nt5, R261Q, and E280K are rare alleles in the European population. R243Q and R270K are minor alleles in the Hispanic group, and were detected only rarely in the European population. Four of the rare mutations in the Hispanic population (A47E, T81P, I102T, and IVS11nt20) are newly described alleles. Mutations in the Hispanic population that were not detected in the European population are A47E, T81P, I102T, D145V, Q304Q, IVS9nt6, and E2/I2(gg-aa).
In seven patients with Armenian heritage, five new mutations were found: E182G, Y387H, A389E, delT323, and del364–368/del198–220. E182G was present on two alleles, and the linked mutations del364–368/del198–220 were present in a homozygous state in two siblings. The mutant alleles IVS10nt3 and IVS10nt546 were also present in the Armenian patients. Two mutant alleles were not detected (Table 1).
Population heterogeneity at the PAH locus.
We further calculated a measurement of population allelic variation at the PAH locus, using the methodology of Guldberg et al. (2) (see “Methods”). Mutation frequencies in a given population were transformed into a number (referred to as the homozygosity j) indicative of a population's heterogeneity. The homozygosity number is inversely proportional to the degree of genetic heterogeneity at the PAH locus in a given population, with a maximum value of 1 being possible (if everyone in a population carries the same allele). The California PKU population as a whole was found to have j= 0.04 (with an 88%PAH allele detection rate). The European (non-Armenian, non-Hispanic) population subset has j= 0.06 (with a 92% allele detection rate). The Hispanic population subset has j = 0.04 (with a 75% allele detection rate), and the Armenian population subset has j= 0.14 (with an 86% allele detection rate).
The homozygosity (j) was also determined for three past studies; the Southeastern US population studied by Eisensmith et al. (12) has j= 0.07 (with 96% allele detection); the German and Danish populations studied by Okano et al. (10) has j= 0.12 (with 64% allele detection); and the German population studied by Trefz et al. (11) has j= 0.23 (with 100% allele detection). The Californian population in the current study clearly has a greater degree of PAH locus heterogeneity versus the above previous reports, with the population studied by Trefz et al. being the most homogenous.
Frequency of mutations ascertained before and after the start ofnewborn screening.
To determine whether changing allele frequencies are related to changes in immigration patterns, the frequency of specific mutant PAH alleles present in late-diagnosed patients (detected based on clinical features, before the inception of newborn screening in California in 1966) was compared with the frequency of mutant PAH alleles present in patients detected by newborn screening after 1966. The major alleles are present at similar frequencies in these two groups (data not shown). R261Q and Y414C occur more frequently after 1966 (current frequencies of 5%, versus 0% before 1966). Because both of these alleles are present in higher frequency in Hispanics, this difference may reflect Hispanic immigration after 1966. It is also possible, inasmuch as these two alleles generally have been associated with a mild clinical course, that a fraction of such patients may have been missed before 1966, when diagnosis was based on phenotype. Conversely, IVS2nt5 (current frequency 0%versus 4% before 1966), L48S (0.8%versus 5%) and del364–368/del198–220 (0%versus 5%) are alleles that were more prevalent before the start of newborn screening and occur more often in the Caucasian population. Although relatively few individuals are involved, both sets of differences may well reflect the effects of Hispanic immigration after 1966.
Frequency of mutant alleles in patients with different IQlevels.
Frequencies of alleles in patients of different IQ categories were determined. We hypothesized that alleles known to cause a more severe biochemical phenotype may be present in greater numbers in poorly controlled patients of low IQ, or in patients diagnosed by phenotype, before the onset of neonatal screening. (We did not expect to find an effect of allele severity on IQ in treated patients with good dietary control of their PHE levels.) For this purpose, we first divided the patient cohorts in age categories and, in each of these age categories, we defined groups with IQ <90, IQ = 90–109, and IQ ≥ 110 (data not shown). Other than for IVS10nt546 (artifactually more frequent in higher IQ category because of contribution from one family), there appears to be no over-representation of a given allele in any IQ category (data not shown). Furthermore, when the rare mutations (present in <2% of the PKU population) are taken collectively, they are not present in higher frequency in any IQ category, compared with their combined frequency in the general California PKU population. The mean IQ at age 5–8 y in patients detected by newborn screening, regardless of dietary control, was 101.9 (n= 49), whereas the mean IQ at age 5–8 y in late-diagnosed patients was 70.9 (n= 22), a clearly significant difference (p< 0.001).
PAH genotype-phenotype analyses.
A lack of concordance between genotype (based on expression analysis) and phenotype was observed when the phenotype chosen for comparison was that of maximal phenylalanine (PHE) level at diagnosis. When the inverse of maximum pretreatment PHE level was plotted versus predicted enzyme activity, no significant correlation was detected between biochemical phenotype and calculated enzyme activity based on genotype (r= 0.32, n= 18) (Fig. 1). Further, no correlation between maximum PHE level and predicted enzyme activity was observed in late-diagnosed patients (r= 0.01) (data not shown). A correlation in the latter group would have been surprising given the variable age at diagnosis. When we considered a number of specific mutations, we found that alleles with low (0–3%) predicted enzyme activity, including R408W, IVS12nt1, E280K, R243X, R252W and F299C, showed biochemical phenotypes in the moderate to severe range, as expected. In contrast, IVS10nt546, a severe mutation with 0% enzyme activity on expression analysis, was relatively evenly distributed over mild, moderate, and severe subgroups based on PHE levels. Conversely, alleles with relatively high (25–50%) predicted activity, including I65T, R261Q, L348V, and Y414C, were present more often in the moderate and severe categories, based on maximum PHE levels at diagnosis (data not shown).
A comparison of the inverse of the maximal diagnosed PHE level (mg/dL, mmol/L values is shown in parentheses) in patients diagnosed by newborn screening (y axis), with the predicted PAH enzyme activity, based on Cos cell expression analysis (x axis). A total of 18 patients had genotypes for which mutant PAH activity for both alleles was known. A significant correlation (at the 5% level of significance) was not found (linear regression analysis:y= 4.9 × 10−4x + 0.04, r= 0.32, n= 18). When the two outlying points (o) are excluded, the correlation changes to r= 0.40 (n= 16), which still is not statistically significant at the 5% level of significance. Previous studies in which similar methodology was used have reported correlation coefficients (r) between 0.74 and 0.91 (10, 12). The power of linear regression to detect a correlation of 0.8 with 95% confidence and a sample size of 18 is 99%.
When IQ levels of patients were compared with predicted enzyme activity via least squares regression analysis, a similar lack of correlation was found in any groups analyzed (data not shown). In the neonatally diagnosed subjects, statistically insignificant Pearson product moment correlations of r= 0.13 (n= 22, eight patients with poor control) and r= 0.08 (n= 23, nine patients with poor control) were obtained when IQ at 5–8 y and when latest IQ, respectively, were compared with predicted PAH activity based on expression analysis (data not shown). An insignificant value of r= −0.04 (n= 14, two patients with poor control) was obtained when latest IQ in subjects diagnosed on phenotype was compared with predicted enzyme activity (data not shown). Furthermore, no correlation was present when IQ at 5–8 y and latest IQ in neonatally diagnosed subjects, and latest IQ in subjects diagnosed on phenotype, were compared with predicted enzyme activity, excluding, in turn, either patients with poor dietary control or those with better control from the analyses (data not shown). In a different tabulation, genotypes were compared with measured IQ by classifying them into mild, moderate, or severe categories based on either predicted or measured enzymatic activity, and using univariate analysis. Again, no significant correlation was detected between predicted genotype severity and measured IQ (data not shown).
DISCUSSION
Since the cloning of the PAH gene in 1985 (26), 328 mutations have been reported (4). In recent years, molecular analysis of the PAH gene has revealed characteristic haplotype and allele frequency patterns in ethnic groups, and within populations (27–36). Studies have suggested a relationship between the frequency of mutant alleles in North American populations and their frequency in ancestral European populations (27–30), and such a relationship may explain the differences in allele frequency observed between Europeans, Hispanics, and Armenians in the present study. Because 29% of the Californian population has Irish, Scottish, or British heritage (Table 2) (37), it is not surprising that the major mutations detected (including R408W, IVS12nt1, IVS10nt546, and I65T) are also those found within the ancestral European populations (28). Although IVS10nt546 is a major mutation in Britain, its actual origin is Anatolian (38).
California has unique demographics, with 25% of residents claiming Hispanic ancestry (Table 2) (37). Alleles that were present in higher frequency in the Hispanic population when compared with the European group include IVS1nt5, R243Q, R261Q, E280K, V388 M, and Y414C. These alleles were also reported in relatively higher frequency in a recent study of the PAH gene in Spain (30). However, significant differences exist between the Californian Hispanic and Spanish populations, possibly due to the Latin American origin of the majority of the Californian Hispanic population. R408W is a major allele in California Hispanics, present in 9% of PKU patients, but it is not present in the ancestral Spanish population. R408W is also a major allele in the non-Hispanic European population, present in 18%. Therefore, whereas the frequency of mutant alleles may be considered to be characteristic of a given ethnic group (39, 40), care is necessary in interpreting the significance of these frequencies in groups that are not genetically isolated.
In the small Armenian subset of the California PKU population, seven of the 14 alleles represent newly identified mutations (including del364–368/del198–220, present in a homozygous state in two siblings), three (IVS10nt3, E182G, and Y386) are present only rarely (<2%) in the California PKU population; two are as yet unidentified; and only one (IVS10nt546) is a major allele (present in >4% of the California PKU population). The majority of PAH mutations identified in the general population are point mutations (88%), and only 12% are deletions (41). One of the 12 identified alleles in the Armenian population is of particular interest, as it contained two linked deletions, del364–368 and del198–220.
Earlier findings suggesting a correlation between genotype and biochemical phenotype generated optimism concerning the predictive capacity of an individual's genotype (10, 12, 14, 41, 43). Eisensmith et al. studied a Southeastern U.S. population from one state and found correlations between the predicted activity of PAH, based on in vitro studies, and pretreatment serum PHE levels (r= 0.841, n= 17) or dietary PHE tolerance (Kendall rank-order coefficient 0.936) (12). In an earlier study of German and Danish subjects, Okano et al. showed that the predicted level of PAH activity correlated strongly with neonatal pretreatment levels of serum PHE and dietary PHE tolerance in both populations (10). The populations studied by Okano et al. were quite homogeneous, with eight mutations accounting for 64% of all mutant PAH alleles (10). Trefz et al. also reported a correlation between predicted PAH activity and pretreatment serum PHE levels in a homogeneous German population (six mutations accounting for 100% of the mutant PAH alleles) (11).
In contrast, the present study—as well as a number of reports from other centers (15–20)—does not confirm a predictive relationship between a patient's genotype and biochemical phenotype in either the neonatally or late-diagnosed subjects. The Californian population is more diverse than the populations analyzed by Eisensmith et al. (12), Okano et al. (10), and Trefz et al. (11), with a larger Hispanic population (25% in California). Using a calculation of population heterogeneity at the PAH locus, the homozygosity number (j) allows for interpopulation comparison to be made quantitatively (2). The higher the value of j, the more homogenous the population with respect to the PAH locus. The California population in the present study has a j of 0.04, the lowest among reported populations (see “Results”). We contend that a heterogeneous population, with a larger number of mutant alleles and a more diverse genetic background, appears to make the correlation between genotype and phenotype less straightforward. Two recent studies also examined the relationship between genotype and biochemical phenotype, but used different methodologies than those used in the current report (44, 45). A meta-analysis study of 365 patients with 73 different PAH mutations classified genotype severity based on in vitro Cos cell expression analyses. Predicted severity was then compared with biochemical phenotypes. The latter were defined by using newborn pretreatment PHE levels, protein loading data, or dietary PHE tolerance. In addition, the in vivo effects of 35 missense mutations present in combination with a known null allele (i.e..“functionally hemizygous” genotypes) were compared with biochemical phenotypes. Although most PAH mutations were found to correspond to the in vivo biochemical phenotype, 11 mutations (15%) were inconsistent in their phenotypic effect. The biochemical phenotype in PKU was concluded to be more complex than would be predicted by the Mendelian inheritance of mutant genotypes at the PAH locus (44). In a multicenter European study of 686 patients with PKU or mild hyperphenylalaninemia, the observed biochemical phenotype was concordant with the predicted phenotype in 79% of the cases, based on a simple model classifying mutant alleles into different severity values (45). Although it was concluded that a simple genotype-phenotype correlation exists in most PKU or mild hyperphenylalaninemia patients (45), such a correlation was not present in 21% of cases.
A number of caveats must be considered before firm conclusions are drawn from a study of genotype-phenotype correlation. The variables under consideration may be affected by the screening process, by the in vitro expression measurements, or by the PHE measurements. It is unlikely that different degrees of correlation are an artifact of the screening process, inasmuch as screening programs are in place in the geographic area of most cohorts studied (10–14).
Inaccurate estimation of PAH activities, with in vitro activity not being representative of actual in vivo function, is possible, but similar expression data were used in studies reporting positive genotype-phenotype correlation (10–14). Variability in plasma PHE concentrations has been reported, with a mean change of 170 μmol/L (2.8 mg/dL) being observed during a 24 h period in 11 PKU patients (46); however, it is unlikely that such a small change could result in a significant error in PHE level ascertainment. PHE determinations in a retrospective versus prospective (10, 12) study may differ, because patients in a prospective study are uniformly fasted for a defined period before collection of PHE levels. However, the differences between “fasting” durations in a study protocol, versus that interval in routine clinical practice in neonates, are likely to be small. Another possible cause of PHE level variability is the nonuniformity of diet in tested neonates. A formula-fed neonate may receive more protein than if breast-fed (47); however, this difference in the newborn period will likely be small. Further, this study covers subjects over a 30-y period, and the trend analysis was uniform over that period.
We therefore believe that the lack of genotype-phenotype correlation reflects the complexity of the genetics of PKU and is not due simply to an artifact of the newborn screening process (48). The Californian population in the present study is more heterogeneous than previously studied populations (10–14), with greater genetic diversity. It is possible that the correlations reported in more homogeneous populations represent the effects of a uniform pool of as yet undefined modifier genes, and not the direct effects of PAH mutations, per se. A study measuring in vivo oxidation of L-[13C]phenylalanine found that genotypes predicted to have either severe or mild PAH function, based on in vitro analysis, in general corresponded to in vivo phenotypes (49). However, markedly different oxidation rates were present in some subjects with identical genotypes, and in vitro activity did not always correlate with in vivo oxidation in others (49). Possible modifying factors of PHE oxidation include different interindividual rates of basal metabolism, synthesis and degradation of PAH protein, and hepatic uptake of circulating PHE (49). Genes responsible for PHE transport across the blood-brain barrier are also candidates for modifying factors in PKU. A lack of correlation between blood and brain PHE concentrations in PKU patients has been documented, using in vivo NMR spectroscopy to measure brain PHE levels (50). In a study of two siblings with identical genotypes, after a PHE load, the patient with the higher IQ and less severe white matter changes on MRI had a lower peak brain PHE level and faster decrease in brain PHE concentration, despite blood PHE levels comparable to those of the more severely affected sibling (51). These studies suggest that interindividual variations in the transport kinetics of PHE across the blood-brain barrier exist and may be important in modifying the clinical phenotype of PKU patients.
The existence of modifier genes was also invoked to explain a lack of correlation between intellectual phenotype and genotype in a study of untreated PKU patients (15). Although a 0% predicted PAH activity corresponded to profound mental retardation in most cases, major differences in IQ were present in some patients with identical genotypes, both in unrelated subjects and within families (15). DiSilvestre et al. found a similar lack of correlation between genotype and intellectual phenotype (16). In the present study, no correlation was found between genotype (based on Cos cell expression studies) and IQ in neonatally or late-diagnosed patients. This was not unexpected, as most patients (40/59 = 68%) had fair to good dietary control, which could potentially modify the effect of severe mutations. (Although we did not find a correlation between genotype and IQ in well-controlled patients, the number of these patients was relatively small, and the statistical analysis must be viewed with caution). By keeping blood PHE levels low, in general, brain PHE levels would also be expected to be lower than if poor dietary control and high blood PHE levels were present. High brain PHE levels may result in an adverse, dose-related effect on brain development, by causing increased myelin turnover and/or decreasing the transport of other essential amino acids into the brain (52).
However, a correlation between genotype and IQ may have been expected in late- diagnosed patients, inasmuch as this group did not have the benefit of early dietary treatment. In late-diagnosed patients, IQ at 5–8 y ranged from 58 to 101 in those with alleles predicted to have 0% activity (n= 6) and from 48 to 77 in those with predicted activity between 12.5% and 14% (n= 3), excluding those with poor dietary control (data not shown). Thus, even in late-diagnosed patients, no correlation was found, although sample size was too small to provide statistically significant conclusions.
In indirect support of the modifier gene hypothesis, Trefz et al. (11) studied a quite homogeneous (j= 0.23), well-treated German PKU population. They found a statistically significant difference between genotype, based upon predicted residual enzyme activity in a Cos cell system, and IQ at age 9 y. Patients who had mutations with a predicted enzyme activity ≥25% demonstrated higher IQs than patients with predicted activities 5–15% (p< 0.04) (11). Patients with PAH mutations predicted to have 0% activity also had lower IQ levels than those with predicted activity ≥ 25%, but the difference was not statistically significant (11). Because the population studied by Trefz et al. was very homogenous, with j= 0.23, it is likely to have a more limited number of potential modifier genes that could affect the ultimate phenotype, and such correlation may be more likely in this instance.
Although the present study did not find a correlation between genotype and clinical phenotype, as defined by PHE level at diagnosis and IQ, the importance of newborn screening was confirmed. The mean IQ at age 5–8 y in patients detected by newborn screening, regardless of dietary control, was 102 (n= 49), a value much higher than the mean IQ at 5–8 y in late-diagnosed patients (71, n= 22). The late-diagnosed patients had poor dietary control initially, supporting the findings of past reports that have detailed the adverse effect of poor dietary compliance on IQ (48, 52–54). However, the mean IQ at age 5–8 y in patients detected by newborn screening with poor dietary control was 99 (n= 15). Although the number of patients is too small to enable one to draw definitive conclusions, it is likely that these patients had good dietary control initially, but poor control later in life, because on average these patients had lower more recent IQs (mean IQ 90, n= 14). Another study did not find an adverse effect on IQ in patients who discontinued dietary therapy (patients were diagnosed neonatally and were on dietary therapy for at least 4 1/2 y) but did report the occurrence of psychopathology (55). Pavone et al. recommended life-long dietary treatment, because the reinstitution of the special diet was found to improve behavioral parameters and the quality of life for patients and their families (56). Koch et al. studied the relationship between dietary control and IQ, academic achievement, marital status, and employment history in 72 adult PKU patients with known genotypes (57). Patients had been diagnosed either neonatally and continued a restrictive diet throughout life (n= 19), neonatally and discontinued the special diet (n= 34), or later in life based on phenotype and continued the PKU diet thereafter (n= 19). A correlation between genotype and IQ in adults was not present, even in those who discontinued the PKU diet. However, continued dietary restriction improved adult achievement, as determined by employment records, marital relationships, and academic achievement (57). Recently, reports have shown that brain MRI white matter changes in adult patients with uncontrolled PKU are partially or completely reversible upon reinstitution of a restricted PHE diet (58, 59). These data reinforce the position that early institution of appropriate dietary therapy is essential, and that dietary therapy may be necessary for life.
The present work underscores the notion that the relationship between an individual's genotype and biochemical and clinical phenotype is complex. Although genotyping may eventually prove valuable in predicting a patient's phenotype, and hence aid in optimizing therapy and determining long-term prognosis, further analysis is needed before firm conclusions can be drawn from an individual's genotype. We recommend that such a cautionary note be incorporated in the genetic counseling of families presenting with newly diagnosed cases of PKU.
Abbreviations
- PKU:
-
phenylketonuria
- PHE:
-
phenylalanine
- PAH:
-
phenylalanine hydroxylase enzyme
- PAH:
-
phenylalanine hydroxylase gene
REFERENCES
Scriver CR, Kaufman S, Eisensmith RC, Woo SLC 1995 The hyperphenylalaninemias. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill, New York, pp 1015–1075
Guldberg P, Levy HL, Hanley WB, Koch R, Matalon R, Rouse BM, Trefz F, de la Cruz F, Henriksen KF, Güttler F 1996 Phenylalanine hydroxylase gene mutations in the United States: report from the maternal PKU collaborative study. Am J Hum Genet 59: 84–94
Eisensmith RC, Woo SLC 1992 Molecular basis of phenylketonuria and related hyperphenylalaninemias: mutations and polymorphisms in the human phenylalanine hydroxylase gene. Hum Mutat 1: 13–23
Nowacki PM, Byck S, Prevosi L, Scriver CR 1998 PAH mutation analysis consortium database, 1997: prototype for relational locus-specific mutation databases. Nucleic Acids Res 26: 220–225
Güttler F 1980 Hyperphenylalaninemia: diagnosis and classification of the various types of phenylalanine hydroxylase deficiency in childhood. Acta Pediatr Scand 280: 7–80
Dhondt JL, Farriauz JP 1981 Hepatic phenylalanine hydroxylase activity in patients with phenylketonuria and hyperphenylalaninemia. J Inherit Metab Dis 4: 59–60
Trefz FK, Bartholome K, Bickel H, Lutz P, Schmidt H, Seyberth HW 1981 In vivo residual activities of the phenylalanine hydroxylating system in phenylketonuria and variants. J Inherit Metab Dis 4: 101–102
Yule KS 1996 Phenylketonuria. In: Jackson PL, Vessey JA (eds) Primary care of the child with a chronic condition. Mosby, St. Louis, MO, pp 623–649
Koch R, Wenz E 1987 Phenylketonuria. Annu Rev Nutr 7: 117–135
Okano Y, Eisensmith RC, Güttler F, Lichter-Konecki U, Konecki DS, Trefz FK, Dasovich M, Wang T, Henriksen K, Lou H, Woo SLC 1991 Molecular basis of phenotypic heterogeneity in phenylketonuria. N Engl J Med 324: 1232–1238
Trefz FK, Burgard P, Konig T, Goebel-Schreiner B, Lichter-Konecki U, Konecki D, Schmidt E, Schmidt H, Bickel H 1993 Genotype-phenotype correlations in phenylketonuria. Clin Chim Acta 217: 15–21
Eisensmith RC, Martinez DR, Kuzmin A, Goltsov AA, Brown A, Singh R, Elsas CJ II, Woo SL 1996 Molecular basis of phenylketonuria and a correlation between genotype and phenotype in a heterogeneous Southeastern US population. Pediatrics 49: 512–516
Güttler F, Henriksen KF, Guldberg P 1996 Phenylalanine tolerance and loading with relation to genotype in phenylalanine hydroxylase deficiency. Int Pediatr 11: 50–53
Svensson E, Iselius L, Hagenfeldt L 1994 Severity of mutations in phenylalanine hydroxylase gene influences phenylalanine metabolism in phenylketonuria and hyperphenylalaninaemia heterozygotes. J Inherit Metab Dis 17: 215–222
Ramus SJ, Forrest SM, Pitt DB, Saleeba JA, Cotton RGH 1993 Comparison of genotype and intellectual phenotype in untreated PKU patients. J Med Genet 30: 401–405
DiSilvestre D, Koch R, Groffen J 1991 Different clinical manifestations of hyperphenylalaninemia in three siblings with identical phenylalanine hydroxylase genes. Am J Hum Genet 48: 1014–1016
Popescu A, Andrian T, Güttler F, Guldberg P 1994 Genotype-phenotype correlation in 11 Romanian PKU families. J Inherit Metab Dis 17: 374–375
Verelst P, Franxois B, Cassiman JJ, Raus J 1993 Heterogeneity of phenylketonuria in Belgium. Dev Brain Dysfunct 6: 97–108
Güttler F, Guldberg P, Henriksen KF 1993 Mutation genotype of mentally retarded patients with phenylketonuria. Dev Brain Dysfunct 6: 92–96
Kunert E, Wolf C, Güttler F, Tyfield LA, Rutland P, Bührdel P, Theile H 1993 Correlation of phenotype and genotype in phenylketonuria patients of the Eastern FRG homozygous for the R408W and R261Q point mutations of the phenylalanine hydroxylase locus. Dev Brain Dysfunct 6: 114–119
Ledley FD, Levy HL, Woo SLC 1986 Molecular analysis of the inheritance of phenylketonuria and mild hyperphenylalaninemia in families with both disorders. N Engl J Med 314: 1276–1280
Guldberg P, Levy HL, Koch R, Berlin CM Jr, Francois BB, Henriksen KF, Güttler F 1994 Mutation analysis in families with discordant phenotypes of phenylalanine hydroxylase deficiency: inheritance and expression of the hyperphenylalaninemias. J Inherit Metab Dis 17: 645–651
Kuzman A, Eisensmith RC, Sergeeva NA, Goltsou AA, Schwartz E, Woo SLC 1995 Complete spectrum of PAH mutations in Tartaria. Eur J Hum Genet 3: 246–255
Griffin RF, Elsas LJ 1975 Classic phenylketonuria diagnosis through heterozygote detection. J Pediatr 88: 512–517
Waters PJ, Parniak MA, Nowacki P, Scriver CR 1998 In vitro expression analysis of mutations in phenylalanine hydroxylase: linking genotype to phenotype and structure to function. Hum Mut 11: 4–17
Kwok SC, Ledley FD, DiLella AG, Robson KJ, Woo SL 1985 Nucleotide sequence of a full-length complementary DNA clone and amino acid sequence of human phenylalanine hydroxylase. Biochemistry 24: 556–561
Eisensmith RC, Goltsov AA, O'Neill C, Tyfield LA, Schwartz EI, Kuzmin AI, Baranovskaya SS, Tsukerman GL, Treacy E, Scriver CR, Güttler F, Guldberg P, Eiken HG, Apold J, Svensson E, Naughten E, Cahalane SF, Croke DT, Cockburn F, Woo SLC 1995 Recurrence of the R408W mutation in the phenylalanine hydroxylase locus in Europeans. Am J Hum Genet 56: 278–86
Scriver CR, John SMW, Rozen R, Eisensmith R, Woo SLC 1993 Associations between populations, phenylketonuria mutations and RFLP haplotypes at the phenylalanine hydroxylase locus: an overview. Dev Brain Dysfunct 6: 11–35
Eisensmith RC, Woo SLC 1994 Population genetics of phenylketonuria. Acta Paediatr Suppl 407: 19–26
Perez B, Desviat LR, Ugorte M 1997 Analysis of phenylalanine hydroxylase gene in the Spanish population: mutation profile and association with intragenic polymorphic markers. Am J Hum Genet 60: 95–102
Rozen R, Mascisch A, Lambert M, Laframboise R, Scriver SC 1994 Mutation profiles of phenylketonuria in Quebec populations: evidence of stratification and novel mutations. Am J Hum Genet 55: 321–326
Goltsov AA, Eisensmith RC, Konecki DS, Lichter-Konecki U, Woo SLC 1992 Associations between mutations and a VNTR in the human phenylalanine hydroxylase gene. Am J Hum Genet 51: 627–636
Avigad S, Kleiman S, Weinstein M, Cohen BE, Schwartz G, Woo SLC, Shiloh Y 1991 Compound heterozygosity in nonphenylketonuria hyperphenylalaninemia: the contribution of mutations for classical phenylketonuria. Am J Hum Genet 49: 393–399
Kang Y, Okano Y, Hase Y, Oura T, Ischiki G 1996 Short tandem repeat polymorphisms in Japanese families with phenylketonuria. J Inherit Metab Dis 19: 375–376
Ounap K, Lillevali H, Klaassen T, Metspalu A, Sitska M 1996 The incidence and characterization of phenylketonuric patients in Estonia. J Inherit Metab Dis 19: 381–382
Velazquez A, Bilbao G, Gonzalez-Trujillo JL, Hernandez D, Perez-Andrade ME, Vela M, Ciceron I, Luera-Luna A, Cederbaum S, Phoenix B 1996 Apparent higher frequency of phenylketonuria in the Mexican state of Jalisco. Hum Genet 97: 99–102
US Census Bureau. US Census Bureau publication CP-2–6 1990 Social and Economic Characteristics, California. US Census Bureau, Washington, DC
Kuzmin AI, Eisensmith RC, Goltsov AA, Sergeeva NA, Schwartz EI, Woo SL 1995 Complete spectrum of PAH mutations in Tartaria: presence of Slavic, Turkic and Scandinavian mutations. Eur J Hum Genet 3: 246–255
Treacy E, Byck S, Clow C, Scriver CR 1993 “Celtic” phenylketonuria chromosomes found: evidence in two regions of Quebec province. Eur J Hum Genet 1: 220–228
Eisensmith RC, Okano Y, Dasovich M, Wang T, Güttler F, Lou H, Guldberg P, Lichter-Konecki U, Konecki DS, Svennson E, Hagenfeldt L, Rey F, Munnich A, Lyonnet S, Cockburn F, Connor JM, Pembrey ME, Smith I, Gitzelmann R, Steinmann B, Apold J, Eiken HG, Giovannini M, Riva E, Longhi R, Romano C, Cerone R, Naughton ER, Mullin SC, Cahalane S, Özalp I, Fekete G, Schuler D, Berencsi GY, Nasz I, Brdicka R, Kamaryt J, Pijackova A, Cabalska B, Boszkowa K, Schwartz E, Kalinin N, Jin L, Chakraborty R, Woo S LC 1992 Multiple origins for phenylketonuria in Europe. Am J Hum Genet 51: 1355–1365
Okano Y, Wang T, Eisensmith RC, Güttler F, Woo SLC 1990 Recurrent mutation in human phenylalanine hydroxylase gene. Am J Hum Genet 46: 919–924
Güttler F, Guldberg R, Henriksen KF, Mikkelesen I, Olsen B, Toft P, Lou H 1992 Optimal planning of dietary therapy based on genotyping of the hyperphenylalaninemic neonate. Enzyme 46: 259
Economou-Petersen E, Henriksen KF, Guldberg P, Güttler F 1992 Molecular basis for nonphenylketonuria hyperphenylalaninemia. Genomics 14: 1–5
Kayaalp E, Treacy E, Waters PJ, Byck S, Nowacki P, Scriver CR 1997 Human phenylalanine hydroxylase mutations and hyperphenylalaninemia phenotypes: a metanalysis of genotype-phenotype correlations. Am J Hum Genet 61: 1309–1317
Guldberg P, Rey F, Zschocke J, Romano V, François B, Michiels L, Ullrich K, Hoffmann GF, Burgard P, Schmidt H, Meli C, Riva E, Dianzani I, Ponzone A, Rey J, Güttler F 1998 A European multicenter study of phenylalanine hydroxylase deficiency: classification of 105 mutations and a general system for genotype-based prediction of metabolic phenotype. Am J Hum Genet 63: 71–79
MacDonald A, Rylance G, Hall SK, Asplin D, Booth IW 1996 Factors affecting the variation in plasma phenylalanine in patients with phenylketonuria on diet. Arch Dis Child 74: 412–417
Tsang RC, Nichols BL 1988 Nutrition during Infancy. Hanley & Belfus, Philadelphia, pp 419–420
Ledley FD 1991 Clinical application of genotypic diagnosis for phenylketonuria: theoretical considerations. Eur J Pediatr 150: 752–756
Treacy EP, Delente JJ, Elkas G, Carter K, Lambert M, Waters PJ, Scriver CR 1997 Analysis of phenylalanine hydroxylase genotypes and hyperphenylalaninemia phenotypes using L-[1-13C]phenylalanine oxidation rates in vivo: a pilot study. Pediatr Res 42: 430–435
Weglage J, Möller HE, Wiedermann D, Cipcic-Schmidt S, Zschocke J, Ullrich K 1998 In vivo NMR spectroscopy in patients with phenylketonuria: clinical significance of interindividual differences in brain phenylalanine concentration. J Inherit Metab Dis 21: 81–82
Weglage J, Wiedermann D, Möller H, Ullrich K 1998 Pathogenesis of different clinical outcomes in spite of identical genotypes and comparable blood phenylalanine concentrations in phenylketonurics. J Inherit Metab Dis 21: 181–182
Smith I, Beasley MG, Ades AE 1990 Effect on intelligence of relaxing the low phenylalanine diet in phenylketonuria. Arch Dis Child 65: 311–316
Fishler K, Azen CG, Henderson R, Friedman EB, Koch R 1987 Psychoeducational findings among children treated for phenylketonuria. Am J Ment Def 92: 65–73
Fisch RO, Chang P-N, Weisberg S, Guldberg P, Guttler F, Tsai MY 1995 Phenylketonuria patients decades after diet. J Inherit Metab Dis 18: 347–353
Waisbren SE, Mahon BE, Schnell PR, Levy HL 1987 Predictions of intelligence quotient change in persons treated for phenylketonuria early in life. Pediatrics 79: 351–355
Pavone L, Meli C, Nigro F, Lisi R, Di Raimondo S, Mollica F 1993 Late diagnosed phenylketonuria patients: clinical presentation and results of treatment. Dev Brain Dysfunct 6: 184–187
Koch R, Fishler K, Azen C, Guldberg P, Güttler F 1997 The relationship of genotype to phenotype in phenylalanine hydroxylase deficiency. Biochem Mol Med 60: 92–101
Cleary MA, Walker JH, Wraith JE, White F, Tyler K, Jenkins JP 1995 Magnetic resonance imaging in phenylketonuria: reversal of cerebral white matter change. J Pediatr 127: 251–255
Ullrich K, Funders B, Weglage J, Hahn-Ullrich H, Koch HG, Moller H, Bick U, Schvierer G, Ludolph A 1995 Magnetic resonance imaging and proton spectroscopy in PKU. Int Pediatr 10: 95–99
Acknowledgements
The authors thank Kathleen Schmidt-Yule, R.N., M.S., Carol Ohnstad, R.D., C.S., Kara Weisiger, M.S., Jan Barry, R.N., Linda Levinson, R.N., and Elisabeth Wenz, R.D., for their helpful comments and care of patients, Karol Fishler, Ph.D., for performing psychological testing, Jiaping Ning, M.S., for performing the univariate statistical analysis, and Faye Eggerding, Ph.D., for her contribution to earlier phases of this work.
Author information
Authors and Affiliations
Additional information
This work was supported by the State of California Newborn Screening Contract #97–10524; by the Children's Hospital of Los Angeles Research Institute; and by National Institutes of Health grant HD 17711 to S.L.C.W. and grant MO1RR01271 to the Pediatric Clinical Research Center, UCSF. G.M.E. was supported by National Institutes of Health Training grant GM 07085.
Rights and permissions
About this article
Cite this article
Enns, G., Martinez, D., Kuzmin, A. et al. Molecular Correlations in Phenylketonuria: Mutation Patterns and Corresponding Biochemical and Clinical Phenotypes in a Heterogeneous California Population. Pediatr Res 46, 594 (1999). https://doi.org/10.1203/00006450-199911000-00017
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199911000-00017
This article is cited by
-
Neonatal screening by DNA microarray: spots and chips
Nature Reviews Genetics (2005)
-
Beyond Mendel: an evolving view of human genetic disease transmission
Nature Reviews Genetics (2002)