Abstract
The aim of the present study was to investigate possible long-term effects of postnatally administered oxytocin on weight gain, gastrointestinal hormone levels, and nociceptive thresholds in rats. For this purpose, s.c. daily injections of oxytocin (1 mg/kg) or saline (NaCl, 0.9%) were given to male and female rat pups on d 10-14 after birth. The animals were killed at the age of 60 or 94 d. Treatment with oxytocin resulted in higher body weight in males, 60 d after birth, and in females from d 60 and throughout the rest of the experiment, compared with controls. The higher body weight was due to an increased weight gain in oxytocin-treated rats, compared with controls, which was most pronounced between 40 and 60 d after birth. Oxytocin-treated male rats had increased circulating levels of cholecystokinin, a tendency to increased plasma levels of insulin (p = 0.066), and relatively more adipose tissue in the thigh and interscapular region, compared with controls. At the age of 60 d, oxytocin-treated female and male rats had a prolonged withdrawal latency when measured in the tail-flick test, compared with controls. This study shows that oxytocin can induce long-lasting changes in weight gain, hormone levels, and nociceptive thresholds, when administered postnatally, in female and male rats.
Similar content being viewed by others
Main
Postnatal growth retardation is associated with an increased morbidity and mortality in the infancy period, and also with an increased risk of developing diseases, such as cardiovascular diseases and non-insulin-dependent diabetes mellitus, later in life(1). Intrauterine growth retardation and premature birth are conditions often related to poor postnatal growth.
In neonatal rats, handling has been shown to cause long-lasting increases in weight gain and nociceptive thresholds(2, 3), and to up-regulate central glucocorticoid receptors, thereby influencing the activity in the hypothalamic-pituitary-adrenal axis(4). Furthermore, massage given to children can decrease urinary cortisol and norepinephrine levels(5). During recent years handling of premature babies with kangaroo care(6) or massage(7, 8), has been shown to have positive physiologic, metabolic, and behavioral effects, as well as to improve postnatal growth(6–11). The mechanism behind these effects is largely unknown but is likely to be induced by a stimulation of somatosensory nerves as well as by other types of sensory stimuli. The kangaroo method has been shown to change the plasma levels of CCK in premature infants(12), indicating that this treatment stimulates vagal nerve activity and possibly, as a consequence, anabolic metabolism in these infants.
Interestingly, the peptide oxytocin is released in response to nonnoxious stimuli, such as stroking, warm temperature, touch, and light pressure(13, 14). Oxytocin is a nonapeptide produced in neurons that orginate in the paraventricular nucleus and project to many brain areas. Oxytocin induces multiple behavioral, physiologic and endocrine effects(15, 16). For example, acute administration of exogenous oxytocin, to rats, influences insulin(17) and CCK(18) levels in blood. Oxytocin has also been shown to influence circulating levels of growth hormone(19). Furthermore, daily injections of oxytocin given during a 5-d treatment period to adult rats can induce long-lasting increases in weight gain and of the above mentioned hormones(20), elevation of nociceptive thresholds(21), and lowering of blood pressure(22). Taken together these data suggest that the effects of handling on weight gain, such as for example in the Kangaroo method or with massage, may involve oxytocin. The aim of the present study was, therefore, to investigate whether daily injections of oxytocin to rat pups for 5 d stimulate weight gain in a long-term perspective. Furthermore, body fatness and metabolic parameters, such as plasma levels of CCK, insulin, and glucose, were assessed, as well as changes in nociceptive thresholds.
METHODS
Experimental animals. Seven pregnant Sprague-Dawley rats were obtained from B & K Universal AB, Sollentuna, Sweden. The animals were single housed in cages in temperature (20 ± 1 °C)- and humidity(45-55%)-controlled rooms illuminated from 0600 to 1800 h. Pelleted food was used with a protein content of 18.5% (Lactamin, Vadstena, Sweden). Water and food were always available ad libitum. The pups were born 1 wk after the dams' arrival to the animal department. The litter was reduced to eight pups per rat, 7 d after birth. The pups were separated from their mother at 22 d of age and kept in cages of four rats. The study was approved by the Stockholm Ethical Committee for Experiments in Animals.
Experimental design. Body weight was recorded every day from 7-8 d of age. The accuracy of the scale (Mettler PE200) was ±0.1 g. The pups were injected, s.c., daily with oxytocin (1 mg/kg) or saline (NaCl, 0.9%), from d 10 to 14 after birth. The dose and number of days of administration were decided based on previous protocols. Subcutaneous injections were chosen, rather than intracerebroventricular injections, as s.c. administration causes less stress. Furthermore, it would have created problems in the performance of surgical procedures on newborn pups. Day 10-14 after birth was chosen as treatment period as the aim was to treat young, newborn pups, but at the same time it was considered important not to disturb the mother-pup interaction in very early life. From d 20 after birth the pups were weighed one or two times a week. Two experiments were performed. The first experiment (experiment I) was performed on male rats (saline treated;n = 5, oxytocin treated; n = 7), which were killed at the age of 60 d. Trunk blood was collected by decapitation, and the adipose tissue was dissected out from the thigh, the abdominal region, and the interscapular region. The second experiment (experiment II) was performed on male (saline treated; n = 8, oxytocin treated; n = 10) but also on female (saline treated; n = 8, oxytocin treated; n = 6) rats. The animals were killed at the age of 94 d to determine whether the effects of oxytocin observed in experiment I remained after 90 d of age. In this experiment, nociceptive thresholds were also measured in the tail-flick test, at d 60 after birth.
Treatment of blood samples. Immediately after decapitation, trunk blood was collected in chilled plastic tubes containing 10 IU/mL heparin(KABI Pharmacia AB, Stockholm, Sweden) and 500 IU/mL aprotinin (Bayer AB, Stockholm Sweden). The samples were centrifuged immediately, and the plasma was harvested and frozen (-20 °C).
Analysis of blood samples. CCK. Before the RIA, peptide was separated from plasma proteins using Sep-Pak C18 cartridges(Waters Associates, Milford, MA). Before determination of CCK the purified plasma samples were dissolved in half of their original volume and thereby concentrated two times. This was later corrected for. CCK was immunoassayed as described by Smedh and Uvnäs-Moberg(23). The CCK standard (Peninsula Laboratories, San Carlos, CA), antiserum OAL-656 (Otsaka Assay Laboratories, Tukoshima, Japan), and 125I-CCK (NEN DuPont, Boston, MA) were used. The limit of detection of the assay was 3.6 pmol/L. The intra- and interassay coefficients of variation were 10 and 12%, respectively.
Insulin. Plasma samples were incubated with antiserum M8309 (Novo Research Institute, Bagsvaerd, Denmark) for 48 h at 4 °C. Then125 I-insulin (NEN DuPont) was added, and incubation was continued for 4 h at 4 °C. For preparation of standard curves, synthetic rat insulin (Novo Research Institute) was used. Standard insulin, antiserum, and125 I-insulin were diluted in 0.02 M veronal buffer, pH 7.5 with 4% BSA. The limit of detection was 0.05 ng/mL. The inter- and intraassay coefficients of variation were 9.6% and 10.1%, respectively.
Glucose. Glucose was analyzed with the GOD-PAP method (Merckotest 3395, Merck, Darmstadt, Germany).
Nociceptive thresholds. Nociceptive reflexes were assessed by immersing the rat's tail into water thermostatically controlled to 51-52°C. The time latency for withdrawal of the tail was recorded. Three consecutive values were registered, and the mean was used for analysis.
Statistical analysis. The results are presented as mean± SD. The effect of oxytocin on body weight and weight gain was tested by a two-way ANOVA, having age, treatment, and the interaction between age and treatment as components of variation. If a significant difference was found, a t test was used to evaluate at what ages there was a significant difference between oxytocin- and saline-treated rats. Differences in hormone levels, body fat, and nociceptive thresholds, between oxytocin- and saline-treated animals, were identified by t test. P values of 0.05 or less were regarded as statistically significant.
RESULTS
Body weight and weight gain. Male rats. Oxytocin-treated male rats had higher body weight on d 50 (experiment I) and 60 (experiments I and II) after birth (Figs. 1A and 2A) and higher weight gain (Figs. 1B and 2B), which was most pronounced around 40-60 d after birth, compared with controls.
Body weight (A) and weight gain (B) of male rats, in experiment I, treated with oxytocin (filled circles) or NaCl(open squares). Values are mean ± SD. Statistical evaluation was performed by a two-way ANOVA. (A) Treatment: F1,100= 1.32, p = 0.28, interaction (age × treatment):F1,100 = 4.37, p = 0.0001; (B) treatment:F1,90 = 9.27, p = 0.012, interaction (age × treatment): F1,90 = 1.31, p = 0.24. Significant(*p < 0.05) difference between oxytocin- and saline-treated rats, assessed by a t test.
Body weight (A) and weight gain (B) of male rats, in experiment II, treated with oxytocin (filled circles) or NaCl(open squares). Values are mean ± SD. Statistical evaluation was performed by a two-way ANOVA. (A) Treatment: F1,192= 3.36, p = 0.086, interaction (age × treatment):F1,192 = 3.48, p = 0.0001; (B) treatment:F1,176 = 4.74, p = 0.045, interaction (age × treatment): F1,176 = 2.77, p = 0.0024. Significant(*p < 0.05, **p < 0.01) difference between oxytocin- and NaCl-treated rats, assessed by a t test.
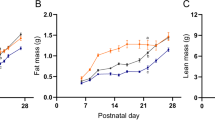
Female rats. Oxytocin-treated females, in experiment II, had higher body weight from d 60 and throughout the rest of the experiment (Fig. 3A) and higher weight gain (Fig. 3B), which was most pronounced around 40-60 d after birth, compared with saline-treated females.
Body weight (A) and weight gain (B) of female rats, in experiment II, treated with oxytocin (filled circles) or NaCl (open squares). Values are mean ± SD. Statistical evaluation was performed by a two-way ANOVA, (A) treatment: F1,144= 5.61, p = 0.036, interaction (age × treatment):F1,144 = 7.23, p = 0.0001; (B) treatment:F1,132 = 8.74, p = 0.012, interaction (age × treatment): F1,132 = 2.14, p = 0.214. Significant(*p < 0.05, **p < 0.01) difference between oxytocin- and NaCl-treated rats, assessed by a t test.
Body fat. Oxytocin-treated rats, in experiment I, had more adipose tissue per g of body weight, in the thigh and interscapular region, compared with saline-treated rats (Fig. 4). No significant difference was observed in the abdominal region.
Weight of adipose tissue in thigh, abdomen, and interscapular region/100 g of body weight, in male rats in experiment I, treated with oxytocin (filled bars) or NaCl (open bars). Values are mean± SD. Statistical evaluation was performed by a t test. Significant (*p < 0.05) from corresponding value for NaCl-treated rats.
CCK, insulin, and glucose. Oxytocin-treated rats, in experiment I, had higher plasma levels of CCK than did saline-treated animals, whereas those on insulin tended to be higher (p = 0.066) and those on glucose lower (p = 0.17) (Fig. 5).
Nociceptive thresholds. Oxytocin-treated males and females, experiment II, had a prolonged withdrawal latency compared with saline-treated control males and females (Fig. 6).
DISCUSSION
This study shows that female and male rats treated with oxytocin s.c., on d 10-14 after birth, have higher body weight, more adipose tissue, increased plasma levels of CCK, and increased nociceptive thresholds in comparison with saline-treated rats at the age of 2-3 mo. The difference in body weight between oxytocin- and saline-treated rats was due to a higher weight gain, in the former group, taking place 40-60 d after birth, i.e. more than 25 d after the last injection of oxytocin. It is not known why the weight gain occurs only during this limited period of time or with such a delay. It is well known from other studies that growth rate and metabolism, in adult life, may be determined by events occurring early in life(24–26). This phenomenon has been called“programming”(1). The mechanisms are largely unknown, but it has been suggested that permanent alterations in gene expression, cell numbers, or sensitivity to hormones may explain the observations(1). The higher weight gain of the oxytocin-treated animals does not coincide with weaning, and consequent access to unlimited amounts of food, but it seems to coincide with puberty and consequently with the rise of circulating growth hormone and steroid hormone levels. The increased circulating levels of CCK and the tendency to increased levels of insulin, as well as the increase in the relative amount of adipose tissue, in oxytocin-treated rats, indicate a stimulated storing of nutrients in the oxytocin-treated group. It may be speculated that the increased CCK levels reflect an increased vagal nerve tone and consequently a stimulated intensity of digestive and/or metabolic activity. The CCK incretin-like effect is of particular interest as it promotes the glucose-induced insulin release(16). A parallel can be drawn to pregnancy, a state characterized by increased circulating basal levels of CCK and insulin, anabolism, and retention of body fat(16). Furthermore, there are studies suggesting that central oxytocin pathways are involved in vagally mediated release of gastrointestinal hormones(16). Administration of oxytocin has also been shown to cause similar metabolic changes in adult rats, as those observed in this study(17, 18). Furthermore, oxytocin has been shown to increase food intake(27), but also to increase weight gain without an increase in food intake(20). Inasmuch as food intake was not measured in this study, it is not known whether food intake was different in the two treatment groups.
About 0.2% of a dose of oxytocin given systemically passes the blood-brain barrier(28), sufficient amounts of oxytocin administered s.c. should therefore have reached the CNS in this study. The effects of oxytocin may therefore be mediated by an action in the CNS.
As mentioned above, it has been suggested that long-term alterations of receptors can occur in response to different environments/treatments in prenatal or early postnatal life. It has, for example, been shown that postnatal injections of vasopressin, to rat pups, amplify vasopressin receptors in adult life(29). In addition, postnatal injections of vasopressin also decrease the amount of oxytocin receptors in the rat (N. Ostrowski, personal communication). Oxytocin and vasopressin differ by only two amino acids (out of nine) and have been shown to have affinity to each others receptors(15). These two peptides are also known to induce opposite effects in many physiologic and experimental situations(15). One possible mechanism by which the postnatal oxytocin injections could have induced the changes observed in adult rats, may, therefore, be by permanent alterations of oxytocin and/or vasopressin receptors.
Oxytocin also caused increased withdrawal latency in the tail-flick test when measured in the adult rat. A long-term increase in nociceptive thresholds has been shown in both male and female adult rats by repeated s.c. oxytocin injections during 5 d(21). This effect could temporarily be reversed by naloxone (an opoid antagonist), suggesting that a change in the endogenous opioid system may have occurred(21). Whether the effect of oxytocin on nociceptive thresholds in the present study also was due to a change in endogenous opioid activity remains to be tested.
The effects of oxytocin treatment observed in this study is very similar to the effects obtained by postnatal handling of rat pups, in which increased nociceptive thresholds(3), increased weight gain(2), and decreased hypothalamic-pituitary-adrenal axis responsivity to stress(4) have been observed. As oxytocin can be released in response to nonnoxious stimuli, such as warmth, touch, and stroking(13, 14), it can be hypothesized that endogenous oxytocin is normally released in the mother-infant interaction, having positive metabolic and endocrine effects on the growing infant. It also may explain why massage and kangaroo care of premature babies have beneficial effects.
Abbreviations
- CCK:
-
cholecystokinin
References
Barker DJP 1994 Mothers, Babies and Disease in Later Life. BMJ Publishing Group, London, pp 1–36
Levine S 1957 Infantile experience and resistance to physiological stress. Science 126: 405
Pieretti S, d'Amore, A, Loizzo A 1991 Long-term changes induced by developmental handling on pain threshold: effects of morphine and naloxone. Behav Neurosci 105: 215–218
Meaney M, Diorio J, Francis D, Widdowson J, LaPlante P, Caldji C, Sharma S, Secki J, Plotsky P 1996 Early environmental regulation of forebrain glucocorticoid receptor gene expression: implications for adrenocortical responses to stress. Dev Neuorsci 18: 49–72
Field T, Morrow C, Valdeon C, Larson S, Kuhn C, Schanberg S 1992 Massage reduces anxiety in child and adolescent psychiatric patients. J Am Acad Child Adolesc Psychiatry 31: 125–131
de Leeuw R, Colin EM, Dunnebier EA, Mirmivava M 1991 Physiological effects of kangaroo care in very small preterm infants. Biol Neonate 59: 149–55
Wheeden A, Scafidi FA, Field T, Ironson G, Valdeon C, Bandstra E 1993 Massage effects on cocaine-exposed preterm neonates. J Dev Behav Pediatr 14: 318–322
Scafidi FA, Field T, Schanberg SM 1993 Factors that predict which preterm infants benefit most from massage therapy. J Dev Behav Pediatr 14: 176–180
Acolet D, Sleath K, Whitelaw A 1989 Oxygenation, heart rate and temperature in very low birth weight infants during skin to skin contact with their mothers. Acta Paediatr Scand 78: 189–193
Karlsson H 1996 Regional skin temperature and dry heat flow: A study of energy loss in newborn babies. Arch Dis Child 75: 130–132
Ludington-Hoe S 1990 Energy conservation during skin-to-skin contact between premature infants and their mothers. Heart Lung 19: 445–451
Törnhage C-J 1997 Plasma somatostatin and cholecystokinin levels in healthy and sick preterm infants from birth up to 2 years of age and in their mothers. PhD thesis, Umeå University, Sweden
Uvnäs-Moberg K, Bruzelius G, Alster P, Lundeberg T 1993 The antinociceptive effect of non-noxious sensory stimulation is partly mediated through oxytocinergic mechanisms. Acta Physiol Scand 149: 199–204
Ågren G, Lundeberg T, Uvnäs-Moberg K, Sato A 1995 The oxytocin antagonist 1-deamino-2-D-Tyr-Oet)-4-Thr-8-Orn-oxytocin reverses the increase in the withdrawal response latency to thermal, but not mechanical nociceptive stimuli following oxytocin administration or massage-like stroking in rats. Neurosci Lett 187: 49–52
Argiolas A, Gessa G L 1990 Central functions of oxytocin. Neurosci Biobehav Rev 15: 217–231
Uvnäs-Moberg K 1994 Role of efferent and afferent vagal nerve activity on behaviour and metabolism during reproduction-integrating function of oxytocin. Psychoneuroendocrinology 19: 687–695
Björkstrand E, Eriksson M, Uvnäs-Moberg K 1996 Evidence of a peripheral and a central effect of oxytocin on pancreatic hormone release in rats. Neuroendocrinology 63: 377–383
Björkstrand E, Ahlénius S, Smedh U, Uvnäs-Moberg K 1996 The oxytocin receptor antagonist 1-deamino-2-D-Tyr(OEt)-4-Thr-8-Orn-oxytocin inhibits effects of the 5-HTIA receptor agonist 8-OH-DPAT on plasma levels of insulin, cholecystokinin and somatostatin. Regul Pept 63: 47–52
Björkstrand E, Hulting A L, Uvnäs Moberg K 1996 Evidence for a dual function of oxytocin in the control of growth hormone secretion in rats. Regul Pept 69: 1–5
Uvnäs-Moberg K, Alster P, Petersson M 1996 Dissociation of oxytocin effects on body weight in two variants of female Sprague-Dawley rats. Integr Physiol Behav Sci 31: 44–55
Petersson M, Alster P, Lundeberg T, Uvnäs-Moberg K 1996 Oxytocin increases nociceptive thresholds in a long-term perspective in female and male rats. Neurosci Lett 212: 87–90
Petersson M, Alster P, Lundeberg T, Uvnäs-Moberg K 1996 Oxytocin causes a long-term decrease of blood pressure in female and male rats. Physiol Behav 60: 1311–1315
Smedh U, Uvnäs-Moberg K 1994 Intracerebroventricularly administered corticotropin releasing factor releases somatostatin through a vagal pathway in freely fed, but not in food-deprived rats. Acta Physiol Scand 151: 241–248
McCarthy R, Fields-Okotcha C 1994 Timing of preweanling maternal effects on development of hypertension in SHR rats. Physiol Behav 55: 839–844
Barraclough CA 1961 Production of anovulatory, sterile rats by single injections of testosterone proprionate. Endocrinology 68: 62–67
Widdowson EM, Mc Cance RA 1963 The effect of finite periods of undernutrition at different ages on the composition and subsequent development of the rat. Proc R Soc Lond Biol 158: 329–342
Björkstrand E, Uvnäs-Moberg K 1996 Central oxytocin increases food intake and daily weight gain in rats. Physiol Behav 59: 947–952
Jones PM, Robinsson IC 1982 Differential clearance of neurophysin and neurohypophyseal peptides from the cerebrospinal fluid in conscious guinea pigs. Neuroendocrinology 34: 297–302
Csaba G, Rónai A, László V, Darvas Z, Berzéteil I 1980 Amplification of hormone receptors by neonatal oxytocin and vasopressin treatment. Horm Metab Res 12: 28–31
Acknowledgements
The authors thank Ferring AB, Malmö, Sweden, for generously supplying oxytocin, and Renée Andersson for skillful technical assistance.
Author information
Authors and Affiliations
Additional information
Supported by the Swedish Medical Research Council (B96-04X-05207-19A).
Rights and permissions
About this article
Cite this article
Uvnäs-Moberg, K., Alster, P., Petersson, M. et al. Postnatal Oxytocin Injections Cause Sustained Weight Gain and Increased Nociceptive Thresholds in Male and Female Rats. Pediatr Res 43, 344–348 (1998). https://doi.org/10.1203/00006450-199803000-00006
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199803000-00006
This article is cited by
-
Maternal and newborn plasma oxytocin levels in response to maternal synthetic oxytocin administration during labour, birth and postpartum – a systematic review with implications for the function of the oxytocinergic system
BMC Pregnancy and Childbirth (2023)
-
Wirkung von Oxytocin auf das menschliche Schmerzerleben
Der Schmerz (2016)
-
Oxytocin Increases Neurite Length and Expression of Cytoskeletal Proteins Associated with Neuronal Growth
Journal of Molecular Neuroscience (2016)
-
Effect of Oxytocin on Neuroblastoma Cell Viability and Growth
Cellular and Molecular Neurobiology (2012)
-
The organizational effects of oxytocin on the central expression of estrogen receptor α and oxytocin in adulthood
BMC Neuroscience (2007)