Abstract
To determine the effects of age, feeding regimen, and antenatal glucocorticoids on intestinal permeability, preterm infants (n = 132) were stratified by gestational age and by diet (mothers' own milk versus preterm formula), and assigned randomly to one of four feeding regimens: early-continuous, early-bolus, standard-continuous, and standard-bolus. At 10, 28, and 50 d of age permeability was determined by measuring the ratio of lactulose/mannitol in the urine after the two sugars were administered enterally for 30 h. The mean (±SE) birth weight and gestational age of the infants were 1044 ± 13 g and 27 ± 0.1 wk, respectively. Permeability changed as a function of age (p = 0.003). Early feeding was associated with a reduction in permeability at 10 d of age (p = 0.01). Antenatal steroid administration was associated with decreased permeability at 28 d of age (p = 0.017). The feeding of human milk (versus formula) was associated with decreased permeability at 28 d of age (p = 0.02). Continuous versus bolus feeding did not affect permeability.
Similar content being viewed by others
Main
The gastrointestinal tract of the preterm infant is underdeveloped compared with that of the term infant. For example, lactase activity, gastrointestinal motor function, and pancreatic function undergo significant maturation in the last trimester (1–3).
Permeability refers to the ability of small molecules to penetrate the gastrointestinal mucosa (4,5). Previous studies have demonstrated that permeability decreases with age in preterm and term infants (6–8). However, these investigations did not study infants sequentially over long periods and/or did not separate the potential effects of feeding on permeability from those of changes with age (6–8). Weaver et al. (9) noted in full-term infants that enteral feeding enhanced the postnatal decline in permeability. An increased permeability is associated with the presence of gastrointestinal mucosal damage, such as that seen in necrotizing enterocolitis (10).
Studies in animals such as the rat have shown that glucocorticoids can accelerate some aspects of gastrointestinal development, i.e. the postnatal decline in gastrointestinal antigen uptake (11). Whether the same is true for the human newborn is unclear. The objective of our study was to determine the effects of age, feeding regimen, antenatal glucocorticoids, and diet on intestinal permeability in preterm infants.
METHODS
Study design. Study infants were part of a prospective feeding trial. They were enrolled within 96 h of birth, stratified by gestational age (26-27 versus 28-30 wk), and by diet (mothers' own milk versus preterm formula), and assigned randomly among four treatment combinations in a balanced two-way design where the two factors were the time of initiation of feeding (early, 4 d versus standard, 15 d) and the method of tube feeding (continuous infusion versus bolus). The four treatment combinations were designated early-continuous, early-bolus, standard-continuous, and standard-bolus.
Study population. Premature infants were recruited based on the following criteria to enroll infants who were likely to survive: 26-30 wk of gestation (as determined by a combination of maternal dates and early antenatal ultrasound), gestational age agreement between the two methods ≤2 wk, appropriate weight for gestational age, postnatal age <96 h, absence of major congenital malformations, and fraction of inspired oxygen <0.60 by 72 h.
The study was approved by the Baylor Institutional Review Board for Human Subject Research. Informed written consent was obtained from parents before enrollment.
Feeding protocol. A feeding schedule was maintained at each infant's bedside so that milk advancement and use of parenteral nutrition were consistent for all study infants. The nutrition protocol was standardized to provide similar intakes of fluid, energy, protein, and minerals among groups. Bolus feedings were given every 3 h. The study was implemented in three phases. The assigned tube-feeding method (continuous versus bolus) was maintained throughout the three phases.
In phase I, conducted from 4 to 14 d after birth, the early group (early-continuous, early-bolus) received 20 mL·kg-1·d-1 of milk and the remaining parenteral nutrition, whereas the standard group (standard-continuous, standard-bolus) received only parenteral nutrition. Infants received either their mothers' own milk or ½-strength Enfamil Premature Formula (Mead Johnson Nutritional Division, Evansville, IN). The composition of parenteral nutrition was standardized during phase I.
In phase II, 15-22 d after birth, all infants received milk (or formula) beginning with 20 mL·kg-1·d-1 and advancing by that amount daily until complete tube feeding was achieved (150 mL·kg-1·d-1). Enfamil Human Milk Fortifier (Mead Johnson Nutritional Division) was added when infants were receiving 100 mL·kg-1·d-1. Formula-fed infants received the full-strength concentration during this phase.
Phase III began the day complete tube feeding was achieved. The respective milk formulations were continued until full oral feeding was attained.
Permeability measurements. Intestinal permeability was determined at 10, 28, and 50 d of age using the method of Weaver et al. (7). This procedure is based upon the differential absorption of lactulose and mannitol (4,5,7). The amounts of lactulose and mannitol that are absorbed depend on the permeability of the small intestine. Because lactulose is a larger molecule than mannitol it permeates to a lesser degree. The greater the permeability, the larger the ratio of lactulose to mannitol. Because neither sugar is metabolized to any significant degree, they are excreted into the urine in the same amount and ratio as they permeate the mucosa (4,5,7). Thus, changes in the ratio of lactulose to mannitol (what is administered versus what is excreted in the urine) are a measure of intestinal permeability (4,5,7). By administering both sugars simultaneously and expressing the results as a ratio of lactulose to mannitol, other factors such as variations in gastric emptying and intestinal transit time, which might alter the amount of lactulose and mannitol appearing in the urine over a set period of time, are obviated (4,5,7).
During phase I (i.e. at the time of the first measurement), in infants who were being fed, 0.2 mL of the lactulose/mannitol solution (0.020 and 0.004 g/mL, respectively) was added to each 1 mL of feeding. In infants who were not being fed at the time, an amount of the lactulose/mannitol solution equal to that received by infants who were fed was administered either via bolus or continuous gavage as appropriate to the infant's randomization. Thus, an attempt was made to ensure that the groups received the same amount of lactulose and mannitol. During phase II and III, 0.2 mL of the lactulose/mannitol solution (0.020 and 0.004 g/mL, respectively) were added to each milliliter of feeding.
The lactulose/mannitol was administered for a total of 30 h (7). During the last 6 h, all urine was collected continuously as previously described (7). Urine was stored at -20°C with 0.1 mL of sodium merthiolate until analyzed (7).
Urine analysis. Lactulose and mannitol in urine and milk were determined using a modification of the method of Catassi et al. (6). In brief, the urine or milk was spun in a 1.5-mL microcentrifuge tube at 3000 rpm for 5 min and then filtered through a 0.2-mm Micro prep disc membrane filter (Bio-Rad Laboratories, Richmond, CA). A 20-µL aliquot of the filtered material was injected onto an Aminex HPX 87C 300 7.8 mm cation-exchange column protected with a precolumn Bio-Rad Carbohydrate Deashing System (Bio-Rad Laboratories) and eluted with degassed pure water at a flow rate of 0.6 mL/min at 85°C. The column effluent was monitored with a differential refractometer (Millipore Corporation, Medford, MA) with the sensitivity setting at 64, scale factor at 25, and internal temperature at 50°C. The column was calibrated using lactulose and mannitol as standards.
To account for any variations in the intake of lactulose and mannitol, the lactulose/mannitol ratio was expressed as the ratio of lactulose/mannitol in the urine versus that in the milk. Thus, the greater the permeability, the greater the urine to milk ratio of lactulose/mannitol.
Data analyses. All data were analyzed as randomized. To determine the effect of the two factors (time of initiation, early versus standard, and method of feeding, continuous versus bolus) and their interaction on permeability, a repeated measures ANOVA and covariance (BMDP5V) was used. χ2 and Mantel-Hantzel tests were used to compare dichotomous variables. Linear regression analyses were used to assess relationships between variables. The data were expressed as mean ± SE.
RESULTS
One hundred and thirty-two infants were studied. The mean (±SE) birth weight and gestational age of the infants were 1044 ± 13 g and 27 ± 0.1 wk, respectively. The mean ages of the infants at the time of the first, second, and third studies were 11.0 ± 0.3 d, 29.0 ± 0.3 d, and 53.0 ± 0.8 d, respectively. There were no differences among groups in birth weight, gestational age, intake of parenteral nutrition, or the age or weight of the infants at the time of the lactulose/mannitol measurements (Table 1). Stratification (26-27 wk versus 28-30 wk of gestational age) did not affect the results, and so the data from all infants (26-30 wk) was combined.
As reflected in Table 1, not all infants had three lactulose/mannitol measurements. Reasons for this included: withdrawal at the parents' request, the infants were unstable clinically, or the attending physician requested that the study not be done. Results from five infants (one study each) gave values that were more than 3 SD greater than the mean for the entire group (132 infants). Therefore, these five values were excluded from the analysis.
There were no differences among the groups in the number of infants whose mothers received antenatal glucocorticoids (Table 1). The number of infants with necrotizing enterocolitis (by radiologic diagnosis of pneumatosis intestinalis plus clinical signs and symptoms and/or at surgery) was similar among the groups (Table 1).
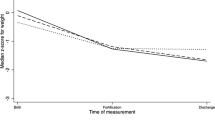
Repeated measures ANOVA demonstrated that permeability increased between 10 and 28 d but then declined by 50 d for the group of infants as a whole (p < 0.01, Fig. 1). The differ in permeability between each of the three ages were significant (Fig. 1).
Early feeding was associated overall with lower permeability compared than that of the standard group (p < 0.01; Fig. 2). The early group had lower permeability compared with that in the standard group at 10 d (Fig. 2). There were no differences between groups at 28 and 50 d of age (Fig. 2). The continuous versus bolus regimens did not affect permeability (data not shown).
Permeability increased with age in both the early and standard feeding groups over the time of the study, but the increase was less in the early group compared with that in the standard group (p < 0.01). Permeability in the early group was less than that in the standard group at 10 d of age. No differences in permeability were noted between groups at 28 and 50 d of age (p < 0.1). At 10 d, n = 31 vs 43; at 28 d, n = 45 vs 45; at 50 d, n = 39 vs 42; early vs standard, respectively.
Repeated measures ANOVA demonstrated that, over the three study periods, antenatal steroid use was associated with an decrease in permeability (p < 0.01; Fig. 3). When the individual studies were analyzed separately, the difference between groups was significant at 28 d (Fig. 3).
We also compared breast milk versus formula with respect to permeability. At 10 d of age there were no differences between groups in permeability (Fig. 4). In contrast, at 28 d of age permeability was lower in infants who were exclusively fed human milk compared with those fed formula (p = 0.02, Fig. 4). By 50 d of age no differences were detected between groups (Fig. 4).
As noted above, there was a relationship between permeability and age (Fig. 1). However, even taking age into account, the relationship between permeability and early feeding, antenatal steroid use, and human milk feeding remained significant.
DISCUSSION
Our data demonstrate that intestinal permeability increases in preterm infants during the first 28 d of life after which it declines (Fig. 1). There are limited data from studies in preterm infants in whom permeability was measured in vitro in the intestinal mucosa (7,8). We measured longitudinal changes in permeability over a longer period than in these previous investigations (7,8). Weaver et al. (7) demonstrated that permeability in infants 27-33 wk of gestational age declined over the first 7 d of age, but they did not measure permeability beyond this time. In contrast to the results of our study (Fig. 1), Beach et al. (8) observed in 26-29-wk gestational age infants that permeability (measured using lactulose and rhamnose) increased between 1-2 wk and 3-4 wk of age and then declined by 4-6 wk to levels comparable to those at 1-2 wk of age. However, they studied only seven infants (8). Additionally, they did not control for the amount of feeding given to the infants during the study period (see below) (8).
When we analyzed our data to determine the effect of early feeding on the development of permeability, we observed that overall, early feeding decreased permeability compared with the standard timing of feeding (Fig. 2). Permeability was lower at 10 d of age in the early group compared with that in the standard group (Fig. 2). Weaver et al. (7) observed no effect of feeding age on permeability but they compared infants fed only within 24 h of birth or after 48 h (7).
Unlike previous studies, we also investigated whether the method of feeding (continuous versus bolus) affects permeability (6–8). No effect of feeding method was observed.
Studies in rats have demonstrated that antenatal administration of steroids can decrease macromolecular uptake in the newborn (12). Thus, we hypothesized that antenatal administration of steroids might also enhance the developmental decline in permeability in the human infant. Our data support this contention (Fig. 3). Antenatal administration of glucocorticoids also is known to enhance other aspects of intestinal development in animals such as the rise in sucrase activity and pancreatic enzyme secretion (12,13). Administration of glucocorticoids speeds up the time at which these functions develop and it is known that there is a lag time between administration and the gastrointestinal response (11–13). Over the 50 d of the study antenatal administration of glucocorticoids was associated with a decline in permeability. However, of the three ages at which it was studied, the differences reached statistical significance only at 28 d (Fig. 3). Changes in intestinal permeability may be relevant clinically as it has been argued that enhanced permeability may increase the susceptibility of the intestine to injury (10).
Infants who were fed human milk exclusively had lower permeability at 28 d than did infants who were fed only preterm infant formula (Fig. 4). No differences were noted between groups at 10 d (Fig. 4). It is possible that there was not enough exposure to feedings at this age to affect permeability. We speculate that by 50 d permeability had declined enough in both groups to obviate differences in permeability between milk- and formula-fed infants. Catassi et al. (6) observed in term infants that those who were milk-fed had lower intestinal permeability than that of formula-fed infants at 7 d of age. These data taken together suggest that postnatal age may indeed influence the ability of milk versus formula to affect permeability.
Although the lactulose/mannitol ratio is a direct measure of intestinal permeability, other factors potentially influence intestinal permeability besides the developmental state of the intestinal mucosa. It has been shown in older children that there is an inverse relationship between urinary lactulose excretion and pancreatic function (14). Thus, early feeding and/or antenatal administration of steroids also may have decreased intestinal permeability by enhancing pancreatic function (14). Indeed, it has been shown that in 32-34-wk gestational age infants that the type of diet can affect the development of pancreatic enzymes (15). In animals such as the rat, antenatal administration of steroids accelerates the development of pancreatic function (13). Further studies are needed to investigate this possibility.
In summary, we have shown that intestinal permeability in the preterm infant born between 26 and 29 wk of gestation increases by 28 d of age and then declines. Early feeding, antenatal administration of steroids, and human milk enhance the decline in intestinal permeability.
Note added in proof: Full details on the prospective feeding trial are available in: Schandler RJ, Schulman RJ, Lau C, Smith EO, Heitkemper MM 1998 Pediatrics (in press).
REFERENCES
Lebenthal E, Lee PC 1980 Development of functional response in human exocrine pancreas. Pediatrics 66: 556–560
Auricchio S, Rubino A, Mürset G 1965 Intestinal glycosidase activities in the human embryo, fetus, and newborn. Pediatrics 35: 944–954
Berseth CL, Nordyke C 1993 Enteral nutrients promote maturation of intestinal motor activity in preterm infants. Am J Physiol 264: G1046–G1051
Travis S, Menzies I 1992 Intestinal permeability: functional assessment and significance. Clin Sci 82: 471–488
Bjarnason I, Macpherson A, Hollander D 1995 Intestinal permeability: an overview. Gastroenterology 108: 1566–1581
Catassi C, Bonucci A, Coppa GV, Carlucci A, Giorgi PL 1995 Intestinal permeability changes during the first month: effect of natural versus artificial feeding. J Pediatr Gastroenterol Nutr 21: 383–386
Weaver LT, Laker MF, Nelson R Intestinal permeability in the newborn. 1984 Arch Dis Child 59: 236–241
Beach RC, Menzies IS, Clayden GS, Scopes JW 1982 Gastrointestinal permeability changes in the preterm neonate. Arch Dis Child 57: 141–145
Weaver LT, Laker MF, Nelson R, Lucas A 1987 Milk feeding and changes in intestinal permeability and morphology in the newborn. J Pediatr Gastroenterol Nutr 6: 351–358
Weaver LT, Laker MF, Nelson R 1984 Enhanced intestinal permeability in preterm babies with bloody stools. Arch Dis Child 59: 280–281
Henning SJ, Rubin D, Shulman RJ 1994 Ontogeny of small intestinal mucosa. In: Johnson LR (ed) Physiology of the Gastrointestinal Tract, Chap. 13. Raven Press, New York, 571–610.
Daniels VG, Hardy RN, Malinowska Nathanielsz PW 1973 The influence of exogenous steroids on macromolecule uptake by the small intestine of the newborn rat. J Physiol 229: 681–695
Lebenthal E, Leung Y-K 1989 Role of Glucocorticoid and thyroxine receptors in the ontogeny of the exocrine pancreas. J Pediatr Gastroenterol Nutr 8: 1–5
Mack DR, Flick JA, Durie PR, Rosenstein BJ, Elis LE, Perman JA 1992 Correlation of intestinal lactulose permeability with exocrine pancreatic dysfunction. J Pediatr 120: 696–701
Zoppi G, Andreotti G, Panjo-Ferrara F, Njal DM, Gaburro D 1972 Exocrine pancreas function in premature and full term neonates. Pediatr Res 6: 880–886
Author information
Authors and Affiliations
Additional information
Supported by the National Institute of Child Health and Human Development, Grant RO-1-HD-28140, the American Gastroenterological Association, SmithKline Beecham Clinical Research Award, the General Clinical Research Center MO1 RR-00188, and the USDA/ARS under Cooperative Agreement No. 58-6250-6-001. This work is a publication of the USDA/ARS Children's Nutrition Research Center, Department of Pediatrics, Baylor College of Medicine and Texas Children's Hospital, Houston, TX. The contents of this publication do not necessarily reflect the views or policies of the USDA, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Rights and permissions
About this article
Cite this article
Shulman, R., Schanler, R., Lau, C. et al. Early Feeding, Antenatal Glucocorticoids, and Human Milk Decrease Intestinal Permeability in Preterm Infants. Pediatr Res 44, 519–523 (1998). https://doi.org/10.1203/00006450-199810000-00009
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199810000-00009
This article is cited by
-
Dilemmas in initiation of very preterm infant enteral feeds—when, what, how?
Journal of Perinatology (2023)
-
Intestinal epithelium in early life
Mucosal Immunology (2022)
-
Evidence-Based Approaches to Minimize the Risk of Developing Necrotizing Enterocolitis in Premature Infants
Current Treatment Options in Pediatrics (2022)
-
The gut microbiota is associated with the small intestinal paracellular permeability and the development of the immune system in healthy children during the first two years of life
Journal of Translational Medicine (2021)
-
Bifidobacterium breve BBG-001 and intestinal barrier function in preterm babies: Exploratory Studies from the PiPS Trial
Pediatric Research (2021)