Abstract
The need for a dietary supply of docosahexaenoic acid (DHA) and arachidonic aid (AA) in term infants was evaluated in a double-masked randomized clinical trial of the effects of supplementation of term infant formula with DHA (0.35% of total fatty acids) or with DHA (0.36%) and AA(0.72%) on visual acuity development. One hundred and eight healthy term infants were enrolled in the study; 79 were exclusively formula-fed from birth (randomized group) and 29 were exclusively breast-fed (gold standard group). Infants were evaluated at four time points during the first 12 mo of life for blood fatty acid composition, growth, sweep visual evoked potential(VEP) acuity, and forced choice preferential looking acuity. Supplementation of term infant formula with DHA or with DHA and AA during the first 4 mo of life yields clear differences in total red blood cell (RBC) lipid composition. Supplementation of term infant formula with DHA or with DHA and AA also yields better sweep VEP acuity at 6, 17, and 52 wk of age but not at 26 wk of age, when acuity development reaches a plateau. The RBC lipid composition and sweep VEP acuity of supplemented infants was similar to that of human milk-fed infants, whereas the RBC lipid composition and sweep VEP acuity of unsupplemented infants was significantly different from human milk-fed infants. Differences in acuity among diet groups were too subtle to be detected by the forced choice preferential looking protocol. Infants in all diet groups had similar rates of growth and tolerated all diets well. Thus, early dietary intake of preformed DHA and AA appears necessary for optimal development of the brain and eye of the human infant.
Similar content being viewed by others
Main
The dietary availability of ω3 and ω6 LCPs may have important effects in developing organs, particularly on the composition of lipid-rich neuronal membranes of the retina and brain(1–4). The last trimester of prenatal development and the early postnatal months represent a period of rapid maturation of the photoreceptors and of rapid increase in the number of synapses and dendritic aborizations in the brain(5–10). These processes require the deposition of lipids, particularly ω3 and ω LCPs, in neuronal membranes. Limitation in the supply of LCPs may modify the growth and function of the CNS. Indeed, the quantity and quality of LCPs incorporated into neural membranes influences their physical and functional properties(3,11–18).
De novo synthesis of ω3 and ω6 fatty acids does not occur in the human; thus, these fatty acids must be obtained in the diet and are considered EFAs. The parent fatty acids of the ω3 and ω6 series are LNA (18:3ω3) and LA (18:2ω6). The ω3 andω6 LCPs, which include EPA (20:5ω3), DHA (22:6ω3), AA(20:4ω6), and docosapentaenoic acid (22:5ω6), can be synthesized from the parent EFAs by desaturation, elongation, and peroxisomal partialβ-oxidation reactions. Differences in plasma and RBC lipid composition between infants fed diets with and without preformed LCPs(19–24) suggest that infants are unable to synthesize DHA from the precursor LNA in sufficient amount to meet accretion needs. Therefore, infants may require a dietary source of preformedω3 LCPs to support normal development of the nervous system. The requirement for a dietary source of preformed ω6 LCP is less clear. The AA content of RBC membranes in infants fed formulas which provide LA but no preformed AA is similar to that observed in human milk-fed infants, indicating that biosynthesis from LA occurs(25–28). In addition, a study with deuterated LA in infants suggests that conversion of labeled LA to AA is severalfold greater than the corresponding DHA biosynthesis from LNA(29). On the other hand, DHA supplementation of preterm formulas using marine oil showed lower AA content in RBCs(19,30). Lower AA has been associated with poorer growth in preterm infants(19,31). Although it is likely that the high EPA present in the marine oil was responsible for the lower AA content in RBCs in these studies, there is preliminary evidence that DHA supplementation with low EPA fish oil may also affect growth in preterm infants(32). Because of the potential for active competition between ω3 and ω6 EFAs and LCPs for elongation/desaturation or incorporation into lipid membranes, there may be an optimal balance between ω3 and ω6 fatty acids for infant diets. Thus, it is not clear whether DHA-supplemented formulas should also contain AA.
During the last trimester of fetal development, DHA and AA are provided by maternal to fetal transfer. Postnatally, breast-fed infants receive a direct supply of DHA and AA; however, LNA and LA are the only polyunsaturated fatty acids provided in U.S. commercial infant formulas. Formula-fed preterm infants, who lack both the placental supply of DHA and AA during the last trimester and a dietary supply postnatally have been shown in randomized clinical trials to have poorer visual function than either preterm infants fed formulas supplemented with DHA or DHA and AA, breast-fed preterm infants, or breast-fed term infants(32–35).
Formula-fed term infants, who comprise approximately 40% of U.S. newborns(36), receive the benefit of maternal to fetal transfer of DHA during the last trimester, but still show lower DHA content of RBCs by several weeks or months postnatally(26,37–42). Thus, term infants also could be at risk for altered visual system development due to the lack of preformed DHA and/or AA in the diet. Indeed, initial studies of formula-fed term infants showed lower acuity than did infants fed human milk or DHA-supplemented formula in some, but not all, studies conducted to date(25,37–40,42). To directly evaluate the need for a dietary supply of DHA and/or AA for term infants, a randomized trial of the effects LCP supplementation of term infant formula on visual acuity development was conducted.
METHODS
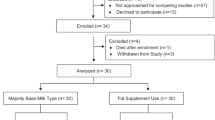
Participants. One hundred and eight healthy term infants were enrolled in the study; 79 were exclusively formula-fed from birth and 29 were exclusively breast-fed from birth through at least 17 wk of age. All were born at 37 to 40 wk of postconceptual age as determined by sonogram, date of last menstrual period, and physical and neurodevelopmental assessment at birth. Only singleton births with birth weights appropriate for gestational age were included. Exclusion criteria were family history of milk protein allergy, genetic or familial eye disease (e.g. hereditary retinal disease, strabismus), vegetarian or vegan maternal dietary patterns, maternal metabolic disease, anemia, or infection, presence of a congenital malformation or infection, jaundice, perinatal asphyxia, meconium aspiration, and any perinatal event which resulted in placement of the infant in the neonatal intensive care unit.
Parents of eligible formula-fed neonates were provided a brief information sheet about the dietary study only after hospital records noted that they had elected to formula-feed. Informed consent was obtained from one or both parents 24-96 h after birth and before the infant's participation. Parents of eligible breast-fed neonates were provided a brief information sheet about the dietary study while inpatients and were contacted by phone 3-5 wk later to determine their interest in participating in the study and whether they planned to continue exclusive breast-feeding through at least 17 wk of age. If a positive response to both questions was obtained, written informed consent was obtained by the Study Coordinator before enrollment. This research protocol observed the tenets of the Declaration of Helsinki and was approved by the Institutional Review Boards of the University of Texas Southwestern Medical Center (Dallas, TX), Presbyterian Medical Center(Dallas, TX), and Medical City Columbia Hospital (Dallas, TX).
Randomization. Formula-fed infants were randomized on the day of enrollment (range = 0-4 d; mean ± SD = 2.1 ± 1.0 d) to one of three diets, described in the paragraph below. Families were recruited from two separate hospitals to encourage ethnic and socioeconomic diversity in the cohort. Infants recruited from both hospitals were randomized using a single randomization schedule at a central location. Each of the three diets was masked by two color/number codes, for a total of six possible diet assignments for each infant. A blocked randomization schedule was developed by the Mead Johnson Research Center and provided in sealed envelopes to the study site.
Diets. Study diets were Enfamil® with iron, Enfamil® with iron supplemented with 0.35% DHA, or Enfamil® with iron supplemented with 0.36% DHA and 0.72% AA. DHA-supplemented and DHA + AA-supplemented formulas contained single cell oils, specifically DHASCO® and ARASCO®(Martek Biosciences, Columbia, MD). The macronutrient and micronutrient content of the formulas was the same as Enfamil® with iron. All formulas were provided as 32-oz ready-to-feed cans, containing 2.2 g of protein, 5.6 g of fat, and 10.3 g of carbohydrate per 100 kcal; other nutrients met existing standards for commercial formula and established American Academy of Pediatrics recommendations. Table 1 provides details of the fatty acid composition of the standard and experimental formulas, along with data obtained from 10 local human milk samples obtained at approximately 6 wk postpartum for comparison. Vitamin E content was adjusted to ensure at least 2 mg of α-tocopherol per g of unsaturated fatty acid in the formulas. Toxicology studies have been completed for the single cell oils used in the enriched formulas; details can be obtained from the manufacturers of the oils (Martek Biosciences, Bethesda, MD) and formula (Mead Johnson Nutritional Research, Evansville, IN)(43–45).
Assigned diets (formula or human milk) were fed exclusively through 17 wk of age. No solid foods were introduced before 17 wk. Beyond 17 wk, formula-fed infants received commercial formula (not provided by the study). The diets of human milk-fed infants varied beyond 17 wk, with some infants weaned to formula at that age, whereas others continued to receive human milk up to 12 mo of age.
General protocol. Infants were evaluated at four time points during the first 12 mo of life for blood fatty acid composition, growth, VEP acuity, and FPL acuity. Table 2 summarizes the schedule of testing. All testing was conducted at a single site. All personnel has been trained in and had considerable experience with the assessment techniques before initiation of the study. Growth measurements were taken by two of six potential independent observers on each visit. VEP and FPL measurements were made by a team of two of seven potential testers;i.e. one investigator with an assistant conducted a single VEP acuity test and a single FPL acuity test. Vision test ages of 6, 17, and 26 wk sample the most rapid period of acuity development(46–49). The vision test age of 52-wk samples a time point when acuity approaches adult levels(46–49). All tests were conducted within ±2 wk of target ages, based on age postconception (e.g. the 17-wk visit was scheduled between 55 and 59 wk postconception). Eighteen month and 4 y follow-up time points are in progress.
Sample size. Sample sizes were estimated using the method described by Rosner(50) for α = 0.05 and 1 -β = 0.80 to detect mean differences in VEP and FPL acuity of 1 SD or greater among diet groups. Using standard deviations of 0.07 logMAR and 0.13 logMAR for term infant sweep VEP acuity and FPL acuity(46–49), the final sample size required at 52 wk is 16 for sweep VEP acuity and 18 for FPL acuity. Anticipating a 25% loss to follow-up over 52 wk, we planned a recruitment of 25 infants for each of the four diet groups and achieved enrollment of 26 to 29 per group. Recruitment and the number of infants completing the protocol at each visit are summarized in Table 3. The greatest loss during follow-up occurred during the initial 6 wk after enrollment, due to the pediatricians' recommendation to switch to a soy protein-based formula after symptoms suggestive of lactose or cow's milk protein intolerance before the 6-wk visit (eight infants), illness of the mother or child unrelated to the protocol (two infants), failure to continue exclusively breast-feeding (one infant), or breast-feeding an infant assigned to a formula diet group (one infant). Of the 96 infants who remained in the protocol to 6 wk of age (the age at which vision was first evaluated), 88 (91.7%) completed the protocol through 12 mo of age.
Blood lipids. Blood samples (2.0 mL) were collected in EDTA vacutainer tubes at birth from cord blood and in microtainer tubes at 17 and 52 wk via heel stick, aided by infant heel warming packs. Plasma and RBCs were separated by centrifugation, lipids were extracted, lipid classes were separated by thin layer chromatography, the fraction was transmethylated with boron trifluoride/methanol, and methylesters were analyzed by capillary gas chromatography using flame ionization detection. Results were obtained as percent total fatty acids and as mass concentration (µg/mL of packed RBCs) based on the addition of an internal standard (10 µg of 23:0 fatty acid). Fatty acid peaks were identified by comparison to GLC68 + 11 standard and using custom software which semiautomated data processing(51).
Inasmuch as the focus of this report is on visual function outcomes, results of fatty acid composition analyses reported are limited to diet-induced differences in major ω3 and ω6 fatty acids in RBC total lipids as indices of compliance to diets and of neural membrane composition(52,53) and, if differences were found, to determine whether they were correlated with visual function outcome.
Growth. Weight was measured using a Healthometer pediatric strain gauge scale accurate to 1 g. Length was measured using Ellard Length Boards accurate to 0.1 cm. Head circumference was measured using a nonstretching tape accurate to 0.1 cm. Subscapular and triceps fat deposition were measured using a Lafayette skin fold caliper accurate to 1 mm. Each length, head circumference, and skin fold measurement was made by two observers, and the average value was used. Absolute values of the differences between observers averaged 0.6% for length measurement (e.g. 0.4 cm at 17 wk), 0.7% for head circumference measurement (e.g. 0.3 cm at 17 wk), and 9.9% for skin fold measurements (e.g. 0.9 mm at 17 wk). Growth data were normalized by expressing them as z scores for term infants of the appropriate age and gender using the nutritional anthropometry software available at the NCHS website (ANTHRO).
Sweep VEP acuity. Stimuli were high contrast (>90%) vertical square-wave gratings, which alternated in counterphase at 6.6 Hz(13.2 contrast reversals/s). Mean luminance was 2.2 log candles/m2. Ten linearly spaced spatial frequencies were presented on each trial in swept parameter protocol, using the NuDiva system developed by Norcia and colleagues(49,54,58), The range of spatial frequencies included in the trial depended on the age of the infant: 0.5 to 8 c/deg at 6 wk, 1 to 15 c/deg at 17 wk, 1 to 20 c/deg at 26 wk, and 2 to 25 c/deg at 52 wk.
Two bipolar placements of Oz versus O1 and O2 were used; gold EEG electrodes were attached to the scalp with electrode cream and Webril pads. The EEG was recorded from the two channels (gain = 10 000, -3 dB cut-off at 1 and 100 Hz) and adaptively filtered(54) in real time to isolate the VEP (397-Hz sampling rate). Amplitude and phase of the response at the second harmonic of the stimulation frequency was calculated for each channel. Noise was measured by determining the amplitude and phase of the two adjacent nonharmonic frequencies.
Grating acuity was estimated with an automated algorithm, which examines signal-to-noise ratio and phase coherence, and performs a linear regression for the final descending limb of the vector averaged function (minimum of three trials; typically five trials) relating VEP second harmonic amplitude (amplitude at the reversal frequency of 13.2 Hz) to spatial frequency.
FPL acuity. Behavioral acuity was estimated using a binocular two-alternative forced choice preferential looking protocol(46). High contrast (>90%) square-wave gratings and paired gray fields (actually 72 c/deg gratings) were rear projected onto two 11.5 deg diameter Polacoat lenscreens (36 deg center-to-center separation). Gratings and gray fields were photometrically matched in mean luminance (2.4 log candles/m2. All test sessions began with a low spatial frequency(0.4 cycles/deg), with approximate 0.5 octave steps between successive stimuli. A 2-down-1-up staircase procedure was used to converge on the acuity threshold in 10 reversals and acuity was estimated by maximum likelihood estimation(55).
Statistical analyses. Data analyses were conducted via two-way repeated measures ANOVA (main effects of diet and age) with multiple comparisons. In all analyses, tests of normality were conducted; parametric ANOVA and pairwise comparisons were conducted if normality tests were satisfied; otherwise, ANOVA by ranks and Dunn's multiple comparisons among groups were conducted. In addition, linear regression was used to examine the relationship between RBC-LCP concentration and visual acuity. Sweep VEP and FPL acuities were expressed in logMAR (log of the minimum angle of resolution;e.g. 20/20 corresponds to a minimum angle of resolution of 1 min arc and logMAR of 0.0, whereas 20/200 corresponds to a minimum angle of resolution of 10 min arc and logMAR of 1.0).
RESULTS
Demographics of the cohort. Demographic information for the cohort as a whole and for the individual diet groups is summarized in Table 4. Ethnic representation in the cohort was similar to that of the greater Dallas area(56): 75% white, 12% black, not of Hispanic origin, 12% Hispanic, and 1% other. Gender representation in the cohort was 55% male and 45% female. Maternal variables included a mean age of 29.1 ± 4.8 y, mean prepregnancy weight of 64.3± 14.8 kg, and mean height of 163.6 ± 5.1 cm. Paternal variables included a mean age of 31.6 ± 5.4 y, mean weight of 81.6± 12.3 kg, and mean height of 179.3 ± 8.2 cm. At least 2 y of college education was completed for 67.6% of mothers and 75.9% of fathers.
Blood lipids. DHA and AA in RBC total lipids for each randomized diet group at each age are summarized in Figure 1. Also shown in Figure 1 are the total RBC-DHA and -AA data for the human milk-fed group for comparison. Statistical analyses of the RBC-DHA and -AA data are summarized in Table 5. At birth, there were no significant differences among groups in RBC-DHA or -AA. By 17 wk of age, the RBCs of infants fed control formula contained significantly lower concentrations of DHA than the two supplemented groups or the human milk group. In addition, the group supplemented with DHA had significantly lower concentrations of AA than the DHA + AA supplemented group or the human milk group. At 52 wk, significantly lower DHA concentrations persisted in the RBCs of infants fed control formula during the first 17 wk of life. In addition, there were significant decreases (p < 0.005) in the concentration of RBC-EPA at 17 wks in formula groups receiving AA supplementation compared with unsupplemented groups.
AA, EPA, and DHA in RBC total lipids(µg/mL of packed RBCs) of infants who were randomly assigned to receive control formula (white bars), DHA-supplemented formula(stipled bars), DHA + AA-supplemented formula (black bars), or who were fed human milk (horizontal lines) during the first 4 mo of life. Vertical bars indicate the standard deviations.
Growth. Z scores for length, weight, and weight-for-length box plots for each of the diet groups are summarized in Figure 2,A-C. All anthropometric outcome data were normally distributed. Using repeated measures ANOVA, no significant main effect of diet was found for weight (F = 0.67, p = 0.57), length (F = 1.11, p = 0.35), or weight-for-length(F = 0.85, p = 0.47). In addition, there were no significant differences among diet groups in measures of head circumference(F = 0.89, p = 0.45), subscapular fat (F = 1.51, p = 0.22), or triceps fat deposition (F = 1.59,p = 0.20).
Anthropometrics (A, weight; B, lenght; and C, weight-for-lenght) of infants who were randomly assigned to receive control formula (left box in each group), DHA-supplemented formula (second box in each group), DHA + AA supplemented formula (third box in each group), or who were fed human milk (right box in each group) during the first 4 mo of life. The horizontal line within each box indicates the median. The boundaries of the boxes show the 25th and 75th percentiles. The vertical bars show the 10th and 90th percentiles.
Sweep VEP acuity. Mean visual acuity for each randomized diet group at each age is summarized in Figure 3. Also shown in Figure 3 is the visual acuity of the human milk-fed group for comparison. The outcome of a repeated measures two-way ANOVA for these data are shown in Table 6. There were significant main effects of diet and of age. Multiple comparisons found that acuity in the control formula group was significantly poorer than in the DHA or DHA + AA groups and significantly poorer than in the human milk-fed group at most ages. There was no significant interaction between age and diet, although, at 26 wk of age, acuities were similar in all groups. The groups who received DHA or DHA + AA showed acuity maturation similar to that of the human milk-fed group throughout the first year of life.
Sweep VEP acuity as a function of diet group during the first year of life: control formula (white bars), DHA-supplemented formula (stipled bars), DHA + AA supplemented formula (black bars), human milk (horizontal lines). The control formula group had significantly poorer acuity than other groups at 6, 17, and 52 wk (see Table 7 for details).Vertical bars indicate the standard deviations.
FPL acuity. Mean visual acuity for each randomized diet group at each age is summarized in Figure 4. Also shown in Figure 4 is the visual acuity of the human milk-fed group for comparison. The outcome of a two-way ANOVA for these data showed a significant main effect of age (F3, 291 = 126.55, p = 0.00000) but no effect of diet (F3, 97 = 1.70, p = 0.171) and no interaction (F3, 291 = 0.611, p = 0.789).
FPL acuity as a function of diet group during the first year of life: control formula (white bars), DHA-supplemented formula (stipled bars), DHA + AA supplemented formula (black bars), human milk (horizontal lines).Vertical bars indicate the standard deviations. No significant differences were found among diet groups.
Linear regression of sweep VEP acuity on RBC lipids. The relationship between total RBC lipid composition at the end of the randomized feeding period (17 wk) and sweep VEP acuity measured during the first year of life is summarized in Table 7. Because sweep VEP acuities were expressed in logMAR, negative regression coefficients indicate that better acuity is associated with a higher concentration of the fatty acid in RBCs, whereas positive regression coefficients indicate that poorer acuity is associated with a higher concentration of the fatty acid in RBCs. The strongest relationship, both in terms of the magnitude of the regression coefficients and the consistency of the relationship throughout the first year of life, is the dependence of better sweep VEP acuity on higher concentration of DHA and higher ω3/ω6 LCP ratios in RBCs.
DISCUSSION
Supplementation of term infant formula with 0.35% DHA or with 0.36% DHA and 0.72% AA during the first 17 wk of life yields clear differences in total RBC lipid composition and in sweep VEP acuity throughout the first year of life. The RBC lipid composition and sweep VEP acuity of supplemented infants was similar to that of human milk-fed infants, whereas the RBC lipid composition and sweep VEP acuity of unsupplemented infants was significantly different from human milk-fed infants. With the caveats that the current study was not designed to fully assess safety issues (rare events could not be detected with these sample sizes) and had sufficient power to assess a 0.9 SD difference in growth (approximately 9% weight, 3% length, and 2.5% head circumference), infants in all diet groups had similar rates of growth and tolerated all diets well. These data are consistent with the failure to find an effect of LCP supplementation on growth in a larger randomized clinical diet which was designed to study this outcome(39).
The differences among diet groups in RBC fatty acid composition and sweep VEP in the current study are similar to, although smaller than, the differences we previously reported for RBC composition and VEP acuity among a group of preterm infants fed diets which provided only trace LNA and no DHA(corn oil), 2.7% LNA and no DHA (soy oil), or 1.4% LNA and 0.35% DHA (soy oil and marine oil) of total fatty acids(34). In the preterm study(34), both the soy oil and the soy/marine oil groups had significantly better VEP acuity than the corn oil group at 17 wk corrected age (57 wk postconception). That the effects of supplementation were more subtle in the present study was expected because: 1) unlike the commercial preterm infant formula used as the control group in the earlier study (corn oil-based), all term formulas in the present study provided ample LNA and the LNA/LA ratio was similar to that of human milk, and 2) unlike preterm infants, term infants have the benefit of maternal to fetal transfer of DHA during the entire last trimester. Despite these differences, term infants did show consistently better visual outcomes when provided with a dietary source of preformed DHA or DHA and AA.
There were no significant differences in sweep VEP acuity between the DHA diet group and the DHA and AA diet group, nor was there any significant relationship between AA and sweep VEP acuity in linear regression analyses. These results suggest that the dietary supply of DHA (and not AA) is the specific factor which is associated with optimal visual acuity maturation.
The difference in sweep VEP acuity between groups with and without a dietary source of DHA is approximately equivalent to one line on an eye chart(e.g. 20/70 versus 20/55 at 17 wk). In the context of clinical ophthalmology, this would be considered a minor, albeit statistically significant, difference. It is important to keep in mind, however, that the motivation for the assessment of visual function in infant nutrition studies is not the detection of gross visual impairment requiring treatment but rather the quantification of subtle differences among diet groups which reflect the developmental course of structure and function in the brain and retina. Thus, even a subtle or transient difference in visual function may provide an important clue to the nutritional requirements of the developing CNS during critical periods of development.
The failure to find a significant difference in sweep VEP acuity at 26 wk of age is likely due to a plateau in development around this age period. There is initial rapid development of VEP acuity during the first 15-20 wk of life, followed by a plateau, which extends from about 25-35 wk, and then a slower period of visual development to adult levels by about 80-100 wk(48–49). A similar plateau in acuity maturation has been observed for FPL and Teller acuity cards(46,47,49,57,58). It is easiest to detect differences among groups during periods of rapid change and most difficult during a developmental plateau. Thus, it was expected that the clearest differences among groups would emerge at the 6-, 17-, and 52-wk time points.
There were no significant differences among diet groups in FPL acuity. This outcome differs from our earlier study of preterm infants(34) and from our earlier comparison of formula-fed and breast-fed term infants(35). The most likely factors contributing to the difference between term and preterm studies are the concentration of LNA and the LA/LNA ratios of the control diet and the relative maturity of the visual system at the time of birth. In the preterm study(34), lower FPL acuity at 17 wk corrected age (57 wk postconception) was found only for the infants fed a corn oil-based diet which provided only trace LNA and no DHA. The average magnitude of differences in sweep VEP acuity among diet groups of 0.1 logMAR in the present term study is smaller than the 0.15-0.2 logMAR effect found in the preterm study. FPL and Teller acuity cards are less sensitive to subtle differences in visual acuity than the sweep VEP. In other single center studies, the SD for normal infants' acuity ranges from 0.12 to 0.6 logMAR(0.3-2.0 octave) for preferential-looking techniques and from 0.03 to 0.15 logMAR (0.1-0.5 octave) for sweep VEP techniques(27,34,35,40,41,46,48,49,57–59). In the present study, the SD for FPL acuity averaged 0.15 logMAR (0.50 octave), whereas the SD for sweep VEP acuity averaged 0.08 logMAR (0.26 octave). Thus, sweep VEP protocols have greater power to detect subtle differences in visual function than preferential-looking protocols.
The effects of dietary supply of DHA on sweep VEP acuity were still present at 52 wk of age, although DHA was provided only during the first 17 wk of life. This result suggests that dietary DHA supply during a critical early period in development leads to persistent changes in the underlying neural structure and/or function. This long-term effect is consistent with differences in cortical visual processing revealed by a random dot stereoacuity test even at 3 y of age(35), by differences in rate of information processing(60,61), and by differences in neurodevelopmental outcomes(62,63) among infants with differences in DHA status early in infancy.
Several other recent studies have demonstrated that supplementation of term infant formula with DHA or DHA and AA results in plasma and RBC lipid composition which matches that of human milk-fed infants(25,38–40). In addition, in nonrandomized studies comparing term infants fed formula which lacks DHA with those fed human milk, lower DHA content is found in cerebral cortex of formula-fed infants(28,64) as well as poorer visual acuity(35,37,41,65); but for exceptions see Innis et al.(27).
In addition to these nonrandomized studies, at least three other randomized clinical trials of the functional effects of dietary supply of DHA and AA on visual acuity have been conducted with term human infants. One of the studies used approximately the same level of DHA supplementation as in our diets and showed significantly better visual acuity in DHA supplemented infants(37). The other two studies used a lower level of DHA or DHA + AA supplementation; one reported a preferential looking visual acuity advantage at early but not later test ages(40), whereas the second reported no advantage in visual maturation associated with DHA or DHA + AA supplementation(39).
There are several differences among the studies that may account for discrepant results. First, there may be a dose-dependent effect. In both the nonrandomized comparisons of human milk-fed and formula-fed infants and the randomized trials of formula supplementation, a wide range of dietary DHA levels have been used (0.1-0.6% of total fatty acids). In general, studies of term infants with dietary DHA levels at ≥0.30% of total fatty acids(35,37,41,65) have reported significant differences in visual function among diet groups whereas, with the exception of one randomized trial(40), studies with DHA levels at ≤0.20% of total fatty acids(27,39,59) have yielded negative results. Second, a wide range of test ages for visual acuity has been used, and studies in which acuity tests were conducted during the developmental plateau (26-40 wk) were much less likely to report significant differences among diet groups than those which conducted acuity tests during the first 4 mo of life. Third, several different psychophysical and electrophysiologic protocols for visual acuity have been used by testers with various degrees of expertise in visual assessment. The SD of measurement varies widely among studies (0.10-1.0 octave), and sample size was not large enough in some studies to detect differences among diet groups which may have been detectable with other protocols.
In summary, consistent with data from other recent studies on erythrocyte fatty acid composition, term infants may be at risk for DHA deficit due to their limited ability to form DHA from LNA during the first weeks or months of life, despite the fact that presently most formulas provide ample amounts of LNA. The present study is the first to show a persistent effect in visual acuity development related to dietary intake of DHA. DHA in human milk represents a direct supply of this essential compound at a time when EFA desaturating/elongating activity may be insufficient to meet actual needs. Although brain and retinal structural lipids have a preferential accretion of LCPs, especially DHA and AA, early dietary intake of DHA and AA appear necessary for optimal development of the human brain and eye.
Abbreviations
- AA:
-
arachidonic acid
- DHA:
-
docosahexaenoic acid
- EFA:
-
essential fatty acid
- EPA:
-
eicosapentaenoic acid
- FPL:
-
forced choice preferential looking
- LA:
-
linoleic acid
- LCP:
-
long chain polyunsaturated fatty acid
- LNA:
-
α-linolenic acid
- RBC:
-
red blood cell
- VEP:
-
visual evoked potential
References
Clandinin M, Chappell J, Leong S 1980 Intrauterine fatty acid accretion rates in human brain: implication for fatty acid requirements. Early Hum Dev 4: 121–130
Clandinin M, Chappell J, Leong S 1980 Extrauterine fatty acid accretion rates in human brain: implication for fatty acid requirements. Early Hum Dev 4: 131–138
Fleisler S, Anderson RE 1983 Chemistry and metabolism of lipids in the vertebrate retina. Prog Lipid Res 22: 79–131
Martinez M 1992 Tissue levels of polyunsaturated fatty acids during early human development. J Pediatr 120: S129–S138
Fulton A, Dodge J, Hansen R, Schremser J, Williams T 1991 The quantity of rhodopsin in young human eyes. Curr Eye Res 10: 977–982
Garey L 1984 Structural development of the visual system of man. Hum Neurobiol 3: 75–80
Hendrickson A 1993 Morphological development of the primate retina. In: Simons K (ed) Early Visual Development, Normal and Abnormal. Oxford University Press, New York, p 287–295
Huttenlocher P, deCourten Ch 1987 The development of synapses in the striate cortex of man. Hum Neurobiol 6: 1–9
Mann I 1950 The Development of the Human Retina. Grune& Stratton, New York, pp 68–135
Blakemore C 1995 Mysteries in the making of the cerebral cortex. In: Bock G, Cardew G (eds) Development of the Cerebral Cortex. John Wiley & Sons, West Sussex, England, pp 1–20
Bourre JM, Francois M, Youyou A 1989 The effects of dietary linolenic acid on the composition of nerve membranes, enzymatic activity, amplitude of electrophysiological parameters, resistance to poisons, and performance of learning tasks in rats. J Nutr 199: 1880–1892
Dratz E, Deese A 1986 The role of docosahexaenoic acid in biological membranes: examples from photoreceptors and model membrane bilayers. In: Simopoulos A, Kifer R, Martin R (eds) Health Effects of Polyunsaturated Fatty Acids in Seafoods. Academic Press, New York, pp 319–351
Foote K, Hrboticky N, MacKinnon M, Innis S 1993 Brain synaptosomal, liver, and red blood cell lipids in piglets fed exclusively on a vegetable oil containing formula with and without fish oil supplements. Am J Clin Nutr 51: 1001–1006
Murphy M 1990 Dietary fatty acids and membrane protein function. J Nutr Biochem 1: 68–79
Stubbs C, Smith A 1984 The modification of mammalian polyunsaturated fatty acid composition in relation to fluidity and function. Biochim Biophys Acta 779: 89–137
Weidmann T, Pates R, Beach J, Salmon A, Brown M 1986 Lipid-protein interactions mediate the photochemical function of rhodopsin. Biochemistry 27: 64–69
Wood J 1990 Essential fatty acids and their metabolites in signal transduction. Biochem Soc Trans 18: 755–786
Neuringer M, Connor W, Lin D, Barstad L, Luck S 1986 Biochemical and functional effects of prenatal and postnatal ω-3 fatty acid deficiency on retina and brain in rhesus monkeys. Proc Natl Acad Sci USA 83: 285–294
Uauy R, Hoffman D, Birch E, Birch D, Jameson D, Tyson J 1994 Safety and efficacy of ω-3 fatty acids in the nutrition of very low birth weight infants: soy oil and marine oil supplementation of formula. J Pediatr 124: 612–620
Innis S, Foote K, MacKinnon M, King O 1990 Plasma and red blood cell fatty acids of low-birth-weigh infants fed their mother's expressed breast milk or preterm infant formula. Am J Clin Nutr 51: 994–1000
Koletzko B, Schmidt E, Bremer H, Haug M, Harzer G 1989 Effects of dietary long-chain polyunsaturated fatty acids on the essential fatty acid status of premature infants. Eur J Pediatr 148: 669–675
Putnam J, Carlson S, DeVoe P, Barness L 1982 The effect of variations in dietary fatty acids on the fatty acid composition of erythrocyte phosphatidylcholine and phosphatidylethanolamine in human infants. Am J Clin Nutr 36: 106–114
Sanders T, Naismith D 1979 A comparison of the influence of breast feeding and bottle feeding on the fatty acid composition or erythrocytes. Br J Nutr 4: 619–623
Uauy R, Birch D, Birch E, Tyson J, Hoffman D 1990 Effect of dietary ω-3 fatty acids on retinal function of very low birth weight neonates. Pediatr Res 28: 485–492
Desci T, Thiel I, Koletzko B 1995 Essential fatty acids in full term infants fed breast milk or formula. Arch Dis Child 72:F23–F28
Hoffman DR, Birch EE, Birch DG, Uauy R, Castaneda Y, Wheaton D 1996 Red blood cell (RBC) fatty acid profiles in term infants fed formulas enriched with long-chain polyunsaturates (LCPs). Invest Ophthalmol Vis Sci 37: S802( abst)
Innis S, Nelson C, Rioux M, King DJ 1994 Development of visual acuity in relation to plasma and erythrocyte ω-6 and ω-3 fatty acids in healthy term gestation infants. Am J Clin Nutr 60: 347–352
Makrides M, Neumann M, Byard R 1994 Fatty acid composition of brain, retina, and erythrocytes in breast- and formula-fed infants. Am J Clin Nutr 60: 189–194
Salem N, Wegher B, Mena P, Uauy R 1996 Arachidonic and docosahexaenoic acids are biosynthesized from their 18-carbon precursors in human infants. Proc Natl Acad Sci USA 93: 49–54
Carlson S, Cooke R, Rhodes P, Peeples J, Werkman S, Tolley E 1991 Long-term feeding of formulas high in linolenic acid and marine oil to very low birth weight infants: phospholipid fatty acids. Pediatr Res 30: 404–412
Carlson S, Werkman S, Peeples J 1993 Arachidonic acid status correlates with first year growth in preterm infants. Proc Natl Acad Sci USA 90: 1073–1077
Carlson S, Werkman S, Tolley E 1996 Effect of long chain n-3 fatty acid supplementation on visual acuity and growth of preterm infants with and without bronchopulmonary dysplasia. Am J Clin Nutr 63: 687–697
Birch D, Birch E, Hoffman D, Uauy R 1992 Retinal development in very-low-birth-weight infants fed diets differing in -3 fatty acids. Invest Ophthalmol Vis Sci 33: 2365–2376
Birch E, Birch D, Hoffman D, Uauy R 1992 Dietary essential fatty acid supply and visual development. Invest Ophthalmol Vis Sci 33: 3242–3253
Birch E, Birch D, Hoffman D, Hale L, Everett M, Uauy R 1993 Breast-feeding and optimal visual development. J Pediatr Ophthalmol Strabismus 30: 33–38
Office of Disease Prevention and Health Promotion 1988 Disease Prevention/Health Promotion-The Facts. U.S. Department of Health and Human Services, Washington, DC
Makrides M, Neumann M, Simmer K, Pater J, Gibson R 1995 Are long-chain polyunsaturated fatty acids essential nutrients in infancy. Lancet 345: 1463–1468
Agostoni C, Riva E, Bellu R, Trojan S, Luotti D, Giovannini M 1994 Effects of diet on the lipid and fatty acid status of full-term infants at 4 months. J Am Coll Nutr 13: 658–664
Auestad N, Montalto M, Hall R, Fitzgerald K, Wheeler R, Connor W, Neuringer M, Connor S, Taylor J, Hartmann E 1997 Visual acuity, erythrocyte fatty acid composition, and growth in term infants fed formulas long chain polyunsaturated fatty acids for one year. Pediatr Res 41: 1–10
Carlson S, Ford A, Werkman S, Peeples J, Koo W 1996 Visual acuity and fatty acid status of term infants fed human milk and formulas with and without docosahexaenoate and arachidonate from egg yolk lecithin. Pediatr Res 39: 882–888
Jorgenson M, Hernell O, Lund P, Holmer G, Fleischer Michaelsen K 1996 Visual acuity and erythrocyte docosahexaenoic acid status in breast-fed and formula-fed term infants during the first four months of life. Lipids 31: 99–105
Ponder D, Innis S, Benson J, Siegman J 1992 Docosahexaenoic acid status of term infants fed breast milk or infant formula containing soy oil or corn oil. Pediatr Res 32: 683–688
Innis S, Hansen J 1996 Plasma fatty acid responses, metabolic effects, and safety of microalgal and fungal oils rich in arachidonic and docosahexaenoic acids in healthy adults. Am J Clin Nutr 64: 159–167
Boswell K, Koskello E, Carl L, Glaza S, Hensen D, Williams K, Kyle D 1996 Preclinical evaluation of single-cell oils that are highly enriched with arachidonic and docosahexaenoic acid. Food Chem Toxicol 34: 585–593
Wilbert S, Burns R, Diersen-Schade D, Kelley C ( 1997 Evaluation of single cell sources of docosahexaenoic acid and arachidonic acid: a 4-week safety study in rats. Food Chem Toxicol 35: 967–974
Birch E, Hale L 1988 Criteria for monocular acuity deficit in infancy and early childhood. Invest Ophthalmol Vis Sci 29: 636–643
Birch E 1989 Visual acuity testing in infants and young children. Ophthalmol Clin North Am 2: 369–389
Norcia AM, Tyler CW 1985 Spatial frequency sweep VEP: Visual acuity during the first year of life. Vision Res 25: 1399–1408
Salomao S, Birch E 1996 Individual growth curves for infant visual acuity measured by sweep-VEP and FPL. Invest Ophthalmol Vis Sci 37:S1067( abstr)
Rosner B 1990 Fundamentals of Biostatistics. PWS-Kent Publishing Co, Boston, MA
Hoffman DR, Uauy R 1992 Essentiality of dietary ω-3 fatty acids for premature infants: plasma and red blood cell fatty acid composition. Lipids 27: 886–895
Connor W, Liu D, Neuringer M 1993 Is the docosahexaenoic acid (DHA, 22:6n3) content of erythrocytes a marker for the DHA content of brain phospholipids?. FASEB J 7:A152( abstr)
Carlson S, Carver J, House S 1986 High fat diets varying in ratios of polyunsaturated to saturated fatty acid and linoleic to linolenic acid: a comparison of rat neural and red blood cell membrane phospholipids. J Nutr 116: 718–726
Norcia A 1993 Improving infant evoked response measurement. In: Simons K (ed) Early Visual Development, Normal and Abnormal. Oxford University Press, New York, pp 536–552
Swanson W, Birch E 1992 Extracting thresholds from noisy data. Percept Psychophys 51: 409–422
1993 Texas State Profile. Woods & Poole Economics, Inc, Washington DC
Salomao S, Ventura D 1995 Large sample population age norms for visual acuities obtained with Vistech-Teller acuity cards. Invest Ophthalmol Vis Sci 36: 657–670
Mayer D, Beiser A, Warner A, Pratt E, Raye K, Lang J 1995 Monocular acuity norms for the Teller acuity cards between ages one month and four years. Invest Ophthalmol Vis Sci 36: 671–685
Innis S, Nelson C, Lwanga D, Rioux M, Walsen P 1996 Feeding formula without arachidonic acid and docosahexaenoic acid has no effect on preferential looking acuity or recognition memory in healthy full-term infants at 9 months of age. Am J Clin Nutr 64: 40–46
Carlson S, Werkman S 1996 A randomized trial of visual attention of preterm infants fed docosahexaenoic acid until two months. Lipids 31: 85–90
Werkman S, Carlson S 1996 A randomized trial of visual attention of preterm infants fed docosahexaenoic acid until nine months. Lipids 31: 91–97
Lucas A, Morely R, Cole T, Gore S 1994 A randomized multicentre study of human milk versus formula and later development in preterm infants. Arch Dis Child 70:F141–F146
Carlson SE 1994 Growth and development of premature infants in relation to ω3 and ω6 fatty acid status. World Rev Nutr Diet 75: 63–69
Farquharson J, Jamieson E, Abbasi K, Patrick W, Logan R, Cockburn F 1995 Effect of diet on the fatty acid composition of the major phospholipids of infant cerebral cortex. Arch Dis Child 72: 198–203
Makrides M, Simmer K, Goggin M, Gibson R 1993 Erythrocyte docosahexaenoic acid correlates with the visual response of healthy term infants. Pediatr Res 33: 425–427
Acknowledgements
Formulas were provided free of charge by Mead Johnson Nutritional Research (Evansville, IN). Important contributions in the conduct of this study were made by Yolanda Castañeda, B.S.N., Diana Wheaton, B.S., Mark Bane, Ph.D., Stephen Nussinowitz, Ph.D., Debbie Cornelius, B.A., and Marianne Woody, B.A. We are grateful for the collaboration of the staff of the Margot Perot Women's and Children's Hospital newborn nursery and postpartum care at Presbyterian Medical Center(Dallas, TX) and of the staff of the newborn nursery and postpartum care at Columbia Hospital Medical City (Dallas, TX). We appreciate the continuing pediatric support of Pediatric Associates, Kaiser Permanente Pediatrics, Woddhill Pediatric Associates, Clinical Associates, North Dallas pediatrics, and Debra Burns, M.D.
Author information
Authors and Affiliations
Additional information
Supported by National Institutes of Health Grant HD22380 and supplementary funding provided by Mead Johnson Nutritional Research (Evansville, IN).
Rights and permissions
About this article
Cite this article
Birch, E., Hoffman, D., Uauy, R. et al. Visual Acuity and the Essentiality of Docosahexaenoic Acid and Arachidonic Acid in the Diet of Term Infants. Pediatr Res 44, 201–209 (1998). https://doi.org/10.1203/00006450-199808000-00011
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199808000-00011
This article is cited by
-
Improvement of Astaxanthin Production in Aurantiochytrium limacinum by Overexpression of the Beta-Carotene Hydroxylase Gene
Applied Biochemistry and Biotechnology (2023)
-
Comparative characterization of three Tetraselmis chui (Chlorophyta) strains as sources of nutraceuticals
Journal of Applied Phycology (2022)
-
Enhanced Production of Astaxanthin without Decrease of DHA Content in Aurantiochytrium limacinum by Overexpressing Multifunctional Carotenoid Synthase Gene
Applied Biochemistry and Biotechnology (2021)
-
VEP estimation of visual acuity: a systematic review
Documenta Ophthalmologica (2021)
-
A comparison of contrast sensitivity and sweep visual evoked potential (sVEP) acuity estimates in normal humans
Documenta Ophthalmologica (2019)