Abstract
Endothelin (ET)-1 is a potent vasoconstrictor peptide that modulates basal pulmonary vascular resistance (PVR) in the normal ovine fetus and contributes to high PVR after chronic intrauterine pulmonary hypertension. Although high PVR is present in premature lambs with severe hyaline membrane disease (HMD), whether ET-1 plays a role in the pathophysiology of experimental HMD is unknown. To test the hypothesis that ET-1 activity contributes to high PVR in the premature lamb with HMD, we studied the hemodynamic effects of a selective ETA receptor antagonist, BQ 123, in 10 animals (gestational age 125 d; 147 d = term). After baseline measurements, animals were intubated, treated with surfactant (Infasurf), and mechanically ventilated with a fraction of inspired oxygen of 1.00 for 8 h. Animals were treated with continuous infusions of either BQ 123 (1 mg/h; treatment group, n = 5) or 1% DMSO (control; n = 5). Plasma ET-1 levels progressively increased during prolonged ventilation with hyperoxia (0.8 ± 0.1 pg/mL, baseline to 6.8 ± 2.5 pg/mL, 8 h, p < 0.05). In comparison with control lambs, BQ 123 treatment caused a sustained reduction in pulmonary vascular resistance (0.55 ± 0.04 mm Hg mL-1 min-1, control versus 0.18± 0.04 mm Hg mL-1 min-1, BQ 123, p < 0.05), increased left pulmonary artery blood flow (70 ± 12 mL/min, control versus 194 ± 28 mL/min, BQ 123, p < 0.05), and increased arterial PaO2 (53 ± 14 mm Hg, control versus 174 ± 71 mm Hg, BQ 123, p < 0.05) 8 h after the onset of ventilation. We conclude that circulating levels of ET-1 increase after delivery of premature lambs with severe HMD, and that selective ETA receptor blockade causes sustained improvement in hemodynamics in severe experimental HMD. These studies suggest that ET-1 contributes to the hemodynamic abnormalities in this model of pulmonary hypertension and severe HMD.
Similar content being viewed by others
Main
After premature birth, respiratory failure is the result of both structural and functional immaturity. Surfactant deficiency is a primary defect in HMD and RDS, and treatment with exogenous surfactant can cause dramatic improvement in oxygenation(1). However, exogenous surfactant results in suboptimal responses in up to 50% of human newborns with RDS, suggesting that other mechanisms may contribute to failure of postnatal adaptation after premature birth(1). Human neonates with severe or fatal RDS often have pulmonary hypertension and poor gas exchange(2,3). Mechanisms that lead to an increase in PVR and the role of pulmonary hypertension in outcome are uncertain.
Premature delivery and ventilation of the ovine fetus has provided a useful animal model for the study of HMD and RDS(4). Severe HMD in premature lambs is characterized by elevated PVR, impaired gas exchange, pulmonary edema, and lung inflammation(4–6). Vasoactive mediators, such as endogenous nitric oxide or ET-1, may contribute to changes in PVR in the fetal lung. Inhaled nitric oxide improves oxygenation, increases pulmonary blood flow without increasing pulmonary edema, lowers PVR, and decreases lung neutrophil accumulation in severe experimental HMD(5,6); however, the role of other vasoactive substances in the pathophysiology of HMD is unknown. ET-1 is a potent vasoconstrictor peptide which modulates basal PVR in the normal late gestation ovine fetus and contributes to high PVR after chronic intrauterine pulmonary hypertension(7–10). ET-1 levels are elevated in the human fetal circulation at 18-24 wk of gestation in comparison with maternal levels(11). However, it is uncertain whether ET-1 contributes to high PVR in experimental HMD after delivery of severely premature lambs.
We hypothesized that ET-1 contributes to high PVR in the prematurely delivered lamb and that its effects are mediated through the ETA receptor. Therefore, using a model of HMD and acute lung injury caused by delivery in the premature fetal lamb, we studied circulating ET-1 levels during prolonged mechanical ventilation with hyperoxia and assessed the effects of a selective ETA receptor blocker on pulmonary hemodynamics in this model of severe HMD.
METHODS
Surgical preparation and physiologic measurements. The following methods have been previously described(5,6,9,10). All procedures and protocols were previously reviewed and approved by the Animal Care and Use Committee at the University of Colorado Health Sciences Center. Ten mixed breed (Columbia-Rambouillet) pregnant ewes at 125 d of gestation (term = 147 d) were fasted 24 h before surgery. Ewes were sedated with i.v. pentobarbital sodium (2-4 g) and anesthetized with 1% tetracaine hydrochloride (3 mg) by lumbar puncture. Ewes were kept sedated with pentobarbital but breathed spontaneously throughout the surgery. Under sterile conditions, a uterine incision was made, and the left forelimb of the fetal lamb was delivered. Polyvinyl catheters were advanced into the ascending Ao and superior vena cava after insertion into the axillary artery and vein. A left axillary to sternal thoracotomy exposed the heart and great arteries. A catheter was inserted into the MPA by direct puncture through purse string sutures. Catheters were guided into position with a 14 or 16 gauge i.v. placement unit (Angiocath; Travenol, Deerfield, IL). Catheters were secured by tightening the purse string suture as the introducer was withdrawn. The MPA catheter was inserted between the ductus arteriosus and the pulmonic valve. A LA catheter was inserted in the medial portion of the left atrial appendage. An ultrasonic flow transducer (6 mm, Transonic Systems Inc., Ithaca, NY) was placed around the LPA to measure blood flow to the left lung. The flow transducer cables were attached to an internally calibrated flowmeter (Transonics, Ithaca, NY) for continuous measurements of LPA flow. The absolute values of flows were determined from phasic blood flow signals obtained during baseline periods, as previously described(8,12,13). A correction factor between end-diastolic flow and the internally calibrated zero point on the Transonics flowmeter was added to the mean flow on the Transonics flowmeter. The value obtained from this method correlates with previously determined measures of LPA flow in the late gestation ovine fetal lung(12). Calculation of resistances are reported as left lung PVR (mm Hg mL-1 min-1 = mean MPA pressure - LAP/LPA flow). Study measurements included PAP, AoP, LAP, LPA flow, and arterial blood gas tensions. The Ao, MPA, and LA catheters were connected to a computer driven system to record pressures and flow (Biopac, Santa Barbara, CA). The pressure transducer was calibrated with a mercury column manometer. Heart rate was determined from phasic pulmonary blood flow tracings. Blood samples for pH, PaCO2, and PaO2 were drawn from the Ao and measured at 39.5°C with a Radiometer OSM-3 blood gas analyzer and hemoximeter (Radiometer, Copenhagen).
Ventilator Study (Fig. 1). After stabilization of physiologic parameters (baseline), pancuronium was administered to the fetus (0.1 mg/kg), the fetal head was exteriorized, and a tracheotomy was performed with placement of an endotracheal tube (3.0-mm internal diameter). All animals were treated with exogenous surfactant(Infasurf, kindly provided by E. A. Eagan, M.D.) at an estimated dose of 3 mL/kg (105 mg of phospholipid/kg) before the first breath. Mechanical ventilation was initiated with a continuous-flow, time-cycled, pressure-limited, neonatal ventilator at the following settings: peak inspiratory pressure, 35 cm H2O; positive end expiratory pressure, 6 cm H2O; rate, 30 breaths/min; inspiratory time, 1.0 s; and FIO2, 1.00. After 30 min of mechanical ventilation, the umbilical cord was ligated, and 20 mL/kg normal saline was infused. BQ 123 (1 mg/h; n = 5) or 1% DMSO (n = 5) were continuously infused in the MPA. A 5% dextrose solution was infused to provide 15 mL/h crystalloid and 1 mg kg-1 h-1 pentobarbital.
Animals were ventilated for 8 h. Mechanical ventilator settings were modified during the course of studies based on results of preductal arterial blood gas samples. The management strategy was guided by target PaCO2 and PaO2 values, with guidelines for ventilator adjustment as described in Figure 1. Arterial blood gas tensions were performed 15-20 min after ventilator changes. For statistical analysis, values for blood gas tensions were recorded every hour after the onset of ventilation. No animal received bicarbonate before 4 h of ventilation. Normal saline (10 mL/kg) was infused for a mean AoP < 30 mm Hg. Dopamine was infused at a rate of 10 µg kg-1 min-1 for a mean AoP < 30 mm Hg unresponsive to normal saline infusion.
Drug preparation. BQ 123 (Alexis Biochemicals, San Diego, CA) was dissolved in DMSO (1 mg in 300 µL). This solution was diluted in 8 mL of normal saline and directly infused into the MPA over 8 h at a rate of 1 mL/h. BQ 123 is a selective ETA receptor antagonist without other known effects on the pulmonary vasculature(17). DMSO(1% in normal saline) was infused at the same rate into control animals.
ET-1 plasma assay. To determine the effect of ventilation on circulating ET-1 levels, 2-mL samples were drawn from the aortic catheter at baseline, and at 1, 4, and 8 h after the onset of ventilation. The samples were collected in tubes of EDTA and spun down immediately at 3000 rpm in a refrigerated centrifuge. Plasma was separated and frozen at -70°C. Plasma levels of ET-1 were measured using a commercial enzyme immunoassay kit (R& D Systems, Inc., Minneapolis, MN). The assay was performed according to the instructions provided with the assay.
Data analysis. Data are presented as means ± 1 SEM. Statistical analysis was performed with the Super ANOVA software package(Abacus Concepts, Berkeley, CA). Comparisons were made using univariate repeated measures analysis of variance by linear contrast analysis. p < 0.05 was considered significant.
RESULTS
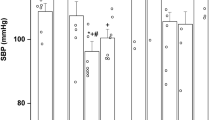
Hemodynamics and gas exchange. There was no difference between the control group and the group treated with BQ 123 at baseline before the onset of ventilation with regards to any hemodynamic variable. After delivery, MPA pressure fell more rapidly in animals treated with BQ 123, but was otherwise not different from controls (Fig. 2;p < 0.05). In contrast to the progressive fall in LPA blood flow during ventilation in control animals, BQ 123 treatment caused sustained increases in LPA flow from 2 to 8 h after delivery (Fig. 2;p < 0.05). PVR was lower in animals treated with BQ 123(Fig. 2;p < 0.05). AoP was not different between the control and the treatment group. At baseline AoP in the control group was 44 ± 1 mm Hg and was 38 ± 3 after 8 h of ventilation. The AoP in the BQ 123 group was 47 ± 3 mm Hg at baseline and was 35 ± 4 after 8 h of ventilation. The ratio of MPA to Ao pressure was lower in the group treated with BQ 123 at 1h (0.87 ± 0.08 versus 0.68 ± 0.08 mm Hg; control versus BQ 123; p < 0.05) and 5 h (0.98 ± 0.03 versus 0.91 ± 0.04 mm Hg; control versus BQ 123; p < 0.05) after delivery. There was no difference in PaCO2 between the two groups; however, BQ 123 treatment caused a sustained increase in PaO2 (Fig. 3; 53± 14 mm Hg, control versus 174 ± 71 mm Hg, BQ 123, p < 0.05 at 8 h). The arterial pH was higher in the BQ 123 treatment group at 3 and 4 h after delivery (Fig. 3; p < 0.05). There was no difference between the groups with regard to peak inspiratory pressure or mean airway pressure during the 8-h ventilator study(Table 1). Positive end expiratory pressure was higher in the control group at 1 h, whereas the ventilator rate was lower in the control group at 5 h. Oxygenation index was higher in the control group than the BQ 123 group at 3, 6, and 7 h after the onset of ventilation.
Hemodynamic effects of prolonged ETA receptor blockade with BQ 123 on mean PAP, LPA blood flow, and left lung PVR after acute delivery and ventilation of premature lambs. BQ 123(1.0 mg/h) lowered mean PAP at 1 h after delivery and caused a sustained increase in LPA flow and fall in PVR after delivery.
Effects of BQ 123 treatment on arterial blood gas tensions for 8 h after acute delivery and ventilation of premature fetal lambs. There was no difference in PaCO2 between the two groups; however, BQ 123 treatment caused a sustained increase in PaO2 from 6 to 8 h after the onset of ventilation. The arterial pH was higher in the BQ 123 treatment group at 3 and 4 h after delivery.
There was no difference in the weight of the animals after the ventilator study (2713 ± 110 g, control versus 2668 ± 143 g, BQ 123). One animal in the control group died 7 h after delivery and one animal in the BQ 123 group died 6 h after delivery. There was no difference in the average amount of sodium bicarbonate given between the two groups (17.6± 7.6 mEq/animal, control versus 7.2 ± 4.5 mEq/animal, BQ 123, p = 0.27). There was no difference in the number of animals receiving dopamine infusion between the two groups (3/5, control versus 2/5, BQ 123). LAP was not different between the two groups at baseline (2.8 ± 0.7 mm Hg, control versus 2.0± 0.8, BQ 123), and during the entire study period.
ET-1 plasma levels. Circulating ET-1 increased in the control group during the 8 h after delivery (Fig. 4;p < 0.05). There was no difference in plasma ET-1 at baseline between the two groups (0.7 ± 0.1 pg/mL, control versus 0.5± 0.1 pg/mL, BQ 123; p < 0.05). There was no increase in plasma ET-1 levels at 1 or 4 h after delivery in the group treated with BQ 123(0.7 ± 0.1 pg/mL, 1 h; 1.2 ± 0.5 pg/mL, 4 h). Due to a technical error, the samples at 8 h in the BQ 123 group were not able to be analyzed.
DISCUSSION
We hypothesized that in premature lambs high PVR and poor gas exchange were due to ET-1 pulmonary vasoconstriction, mediated by increased ETA receptor activity. We found that plasma ET-1 levels increased after delivery and ventilation of premature fetal lambs, and that BQ 123, an ETA receptor antagonist, increased left pulmonary artery blood flow, decreased PVR, and improved oxygenation after premature delivery and prolonged ventilation. These studies suggest that ET-1 contributes to the hemodynamic abnormalities in this model of pulmonary hypertension and severe HMD.
ET-1 is a potent vasoactive peptide with mitogenic effects on vascular smooth muscle, and is produced primarily by the vascular endothelium in the normal lung circulation(14). In the normal fetal lung, ET-1 is present and contributes to high PVR(10,15–17). We have previously shown that chronic intrauterine pulmonary hypertension causes the loss of ETB-mediated vasodilation, progressive ETA-mediated vasoconstriction, and increased lung ET-1 content(7). Furthermore, ET-1 contributes to a model of pulmonary hypertension caused by ligation of the ductus arteriosus in the late gestation ovine fetus. The ETA receptor antagonist, BQ 123, attenuated the in utero increases in PAP, the abnormal transition of the fetus with pulmonary hypertension after delivery, as well as the right ventricular hypertrophy and smooth muscle changes in chronic pulmonary hypertension caused by ligation of the ductus arteriosus in fetal lambs(9).
The physiologic role of ET-1 in the normal ovine fetal lung has been controversial. ET-1 is present in the perinatal lung(15) and is vasoactive in the fetus(8,10,19). Brief infusion of ET-1 causes potent vasodilation acutely(18–21); however, with prolonged infusion, hypertension prevails(18). However, exogenous infusion of ET-1 may not accurately describe the hemodynamic effects of endogenous production of ET-1 in the fetal lung. Evidence suggests that ET-1 acts as a local autocrine and paracrine factor rather than a circulating hormone, because secretion of ET-1 by endothelial cells is polar and directed abluminally toward the interstitial region(22). Some studies of exogenous infusion of ET-1 have emphasized that the major effect of ET-1 in the normal late gestation fetal lung is vasodilation and that the majority of ET-1 receptor activation in the ovine fetal lung are the ETB1 receptors(20), which mediate only vasodilation(10). In contrast, several studies suggest that the ETA receptors play an important role in mediating vasoconstriction in the late gestation ovine fetus(8,10,17). Intrapulmonary infusion of big-ET-1, the precursor to ET-1, causes progressive and sustained pulmonary hypertension without even transient vasodilation(10), suggesting that stimulation of endogenous ET-1 may have very different effects than brief exogenous infusions of ET-1. Recent studies have shown that combined ETA and ETB receptor blockade with Ro 47-0203 does not change the increase in pulmonary blood flow or decrease in pulmonary vascular resistance with in utero oxygen ventilation, suggesting that endogenous ET-1 activity does not play a major role in the increased pulmonary blood flow during the normal transitional circulation at birth(23).
These findings are interesting as little is known of the role of ET-1 and its receptors in pulmonary vasoregulation in the premature fetus and in this model of severe HMD. Past studies of this experimental model of severe HMD suggests that high PVR is partly due to structural changes and an imbalance in production and responsiveness to vasodilator stimuli(4,5,6,24). Pulmonary immaturity leads to surfactant deficiency and respiratory failure, which in part may be improved by exogenous surfactant therapy(1). However, some newborns born prematurely may continue to deteriorate and develop pulmonary hypertension(2,24). Inhaled nitric oxide improves oxygenation, increases pulmonary blood flow without increasing pulmonary edema, lowers PVR, and decreases lung neutrophil accumulation in severe experimental HMD(5,6). The present study suggests that ET-1 also contributes to the development of pulmonary hypertension in experimental HMD. The mechanism by which BQ 123 improved oxygenation after delivery and ventilation of the premature lamb remains uncertain. We speculate that improved pulmonary blood flow and diminished PVR leads to decreased right to left shunting within the lungs or the foramen ovale, thus improving oxygenation. During the ventilator study the pulmonary to aortic pressure ratio was lower in the BQ 123 group at 1 and 5 h, and tended to be lower during the remainder of the study. Likewise, improved right ventricular function by reducing right ventricular afterload may lead to improved cardiac output and arterial and mixed venous oxygen tension.
Although ET-1 was originally thought to contribute only to vasoconstriction and smooth muscle proliferation, accumulating evidence indicates that ET-1 also contributes to inflammation and acute lung injury(25). ET-1 may act as a cytokine(26), promote neutrophil adhesion(27), and increase lung vascular permeability(28). Similarly, the pathophysiology of HMD is also characterized by increased lung vascular permeability and lung neutrophil accumulation(5), as well as altered production of inflammatory cytokines(29). Circulating ET-1 levels are found in humans with acute lung injury and the adult respiratory distress syndrome(30,31). The role of ET-1 in acute lung injury in this model of severe respiratory failure caused by premature delivery and HMD in the ovine fetus remains uncertain.
In summary, chronic blockade of the ETA receptor with BQ 123 lowered PVR, enhanced vasodilation, and improved oxygenation at delivery in the premature lamb. Circulating ET-1 increased after delivery of these animals with severe HMD. These findings support the hypothesis that increased ET and enhanced ETA receptor activity contributes to high PVR in severe experimental HMD. We conclude that ET contributes to pulmonary hypertension in severe HMD.
Abbreviations
- Ao:
-
aorta
- AoP:
-
aorta pressure
- ET:
-
endothelin
- HMD:
-
hyaline membrane disease
- LA:
-
left atrium
- LAP:
-
left atrium pressure
- LPA:
-
left pulmonary artery
- MPA:
-
main pulmonary artery
- PAP:
-
pulmonary artery pressure
- Pao2:
-
arterial partial pressure of oxygen
- Paco2:
-
arterial partial pressure of carbon dioxide
- Fio2:
-
fractional concentration of inspired oxygen
- PVR:
-
pulmonary vascular resistance
- RDS:
-
respiratory distress syndrome
References
Jobe AH 1992 Surfactant in the perinatal period. Early Hum Dev 29: 57–62
Walther FJ, Benders MJ, Leighton JO 1992 Persistent pulmonary hypertension in premature neonates with severe respiratory distress syndrome. Pediatrics 90: 899–904
Skinner JR, Boys RJ, Hunter S, Hey EN 1992 Pulmonary and systemic arterial pressure in hyaline membrane disease. Arch Dis Child 67: 366–373
Jobe A, Ikegami M, Jacobs H, Jones S 1984 Surfactant and pulmonary blood flow distributions following treatment of premature lambs with natural surfactant. J Clin Invest 73: 848–856
Kinsella JP, Parker TA, Galan H, Sheridan BC, Halbower AC, Abman SH 1997 Effects of inhaled nitric oxide on pulmonary edema and lung neutrophil accumulation in severe experimental hyaline membrane disease. Pediatr Res 41: 457–463
Kinsella JP, Ivy DD, Abman SH 1994 Inhaled nitric oxide improves gas exchange and lowers pulmonary vascular resistance in severe experimental hyaline membrane disease. Pediatr Res 36: 402–408
Ivy DD, Ziegler JW, Dubus MF, Fox JJ, Kinsella JP, Abman SH 1996 Chronic intrauterine pulmonary hypertension alters endothelin receptor activity in the ovine fetal lung. Pediatr Res 39: 435–442
Ivy DD, Kinsella JP, Abman SH 1996 Endothelin blockade augments pulmonary vasodilation in the ovine fetus. J Appl Physiol 81: 2481–2487
Ivy DD, Parker TA, Ziegler JW, Galan HL, Kinsella JP, Tuder RM, Abman SH 1997 Prolonged endothelin A receptor blockade attenuates chronic pulmonary hypertension in the ovine fetus. J Clin Invest 99: 1179–1186
Ivy DD, Kinsella JP, Abman SH 1994 Physiologic characterization of endothelin A and B receptor activity in the ovine fetal pulmonary circulation. J Clin Invest 93: 2141–2148
Malamitsi-Puchner A, Antsaklis A, Economou E, Mesogitis S, Papantoniou N, Koutra N, Aravantinos D 1995 Endothelin 1-21 plasma levels in fetuses at 18-24 wk of gestation. J Perinat Med 23: 321–325
Lewis AB, Heymann MA, Rudolph AM 1976 Gestational changes in pulmonary vascular responses in fetal lambs in utero. Circ Res 39: 536–541
Lock JE, Hamilton F, Luide H, Coceani F, Olley PM 1980 Direct pulmonary vascular responses in the conscious newborn lamb. J Appl Physiol 48: 188–196
Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T 1988 A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332: 411–415
MacCumber MW, Ross CA, Glaser BM, Snyder SH 1989 Endothelin: visualization of mRNAs by in situ hybridization provides evidence for local action. Proc Natl Acad Sci USA 86: 7285–7289
Nakamura T, Kasai K, Konuma S, Emoto T, Banba N, Ishikawa M, Shimoda S 1990 Immunoreactive endothelin concentrations in maternal and fetal blood. Life Sci 46: 1045–1050
Wang Y, Coceani F 1992 Isolated pulmonary resistance vessels from fetal lambs. Contractile behavior and responses to indomethacin and endothelin-1. Circ Res 71: 320–330
Chatfield BA, McMurtry IF, Hall SL, Abman SH 1991 Hemodynamic effects of endothelin-1 on ovine fetal pulmonary circulation. Am J Physiol 261:R182–R187
Wong J, Vanderford PA, Fineman JR, Chang R, Soifer SJ 1993 Endothelin-1 produces pulmonary vasodilation in the intact newborn lamb. Am J Physiol 265:H1318–H1325
Wong J, Fineman JR, Heymann MA 1994 The role of endothelin and endothelin receptor subtypes in regulation of fetal pulmonary vascular tone. Pediatr Res 35: 664–670
Cassin S, Kristova V, Davis T, Kadowitz P, Gause G 1991 Tone-dependent responses to endothelin in the isolated perfused fetal sheep pulmonary circulation in situ. J Appl Physiol 70: 1228–1234
Wagner OF, Christ G, Wojta J, Vierhapper H, Parzer S, Nowotny PJ, Schneider B, Waldhausl W, Binder BR 1992 Polar secretion of endothelin-1 by cultured endothelial cells. J Biol Chem 267: 16066–16068
Winters JW, Wong J, Van Dyke D, Johengen M, Heymann MA, Fineman JR 1996 Endothelin receptor blockade does not alter the increase in pulmonary blood flow due to oxygen ventilation in fetal lambs. Pediatr Res 40: 152–157
Stahlman M, Blankenship WJ, Shepard FM, Gray J, Young WC, Malan AF 1972 Circulatory studies in clinical hyaline membrane disease. Biol Neonate 20: 300–320
Michael JR, Markewitz BA 1996 Endothelins and the lung. Am J Respir Crit Care Med 154: 555–581
McMillen MA, Sumpio BE 1995 Endothelins: polyfunctional cytokines. J Am Coll Surg 180: 621–637
Lopez Farre A, Riesco A, Espinosa G, Digiuni E, Cernadas MR, Alvarez V, Monton M, Rivas F, Gallego MJ, Egido J 1993 Effect of endothelin-1 on neutrophil adhesion to endothelial cells and perfused heart. Circulation 88: 1166–1171
Rodman DM, Stelzner TJ, Zamora MR, Bonvallet ST, Oka M, Sato K, O'Brien RF, McMurtry IF 1992 Endothelin-1 increases the pulmonary microvascular pressure and causes pulmonary edema in salt solution but not blood-perfused rat lungs. J Cardiovasc Pharmacol 20: 658–663
Jones CA, Cayabyab RG, Kwong DYC, Stotts C, Wong B, Hamdan H, Minoo P, deLemos RA 1996 Undetectable interleukin (IL)-10 and persistent IL-8 expression in early hyaline membrane disease: a possible developmental basis for the predisposition to chronic inflammation in preterm newborns. Pediatr Res 39: 966–975
Druml W, Steltzer H, Waldhausl W, Lenz K, Hammerle A, Vierhapper H, Gasic S, Wagner OF 1993 Endothelin-1 in adult respiratory distress syndrome. Am Rev Respir Dis 148: 1169–1173
Langleben D, DeMarchie M, Laporta D, Spanier AH, Schlesinger RD, Stewart DJ 1993 Endothelin-1 in acute lung injury and the adult respiratory distress syndrome. Am Rev Respir Dis 148: 1646–1650
Author information
Authors and Affiliations
Additional information
Supported by The Children's Hospital Research Institute Career Development Award (D.D.I., J.P.K.), the National Institutes of Health Grants H241012 and 46481 (S.H.A.), the March of Dimes Birth Defects Foundation (D.D.I., J.P.K.), the Bugher Physician-Scientist Training Program (D.D.I.), the American Heart Association Established Investigator Award (S.H.A.), and General Clinical Research Centers Program, National Centers for Research Resources, National Institutes of Health Grant M0100069.
Rights and permissions
About this article
Cite this article
Ivy, D., Parker, T., Kinsella, J. et al. Endothelin A Receptor Blockade Decreases Pulmonary Vascular Resistance in Premature Lambs with Hyaline Membrane Disease. Pediatr Res 44, 175–180 (1998). https://doi.org/10.1203/00006450-199808000-00006
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199808000-00006
This article is cited by
-
Factors relating caesarean section to persistent pulmonary hypertension of the newborn
World Journal of Pediatrics (2017)
-
Persistent pulmonary hypertension of the newborn with transposition of the great arteries: successful treatment with bosentan
European Journal of Pediatrics (2008)