Abstract
Rhabdomyosarcoma (RMS) cells express the polysialylated (PSA) form of the neural cell adhesion molecule (NCAM). During embryogenesis, PSA-NCAM is widespread and dynamically regulates embryonal developing processes, whereas postnatally, PSA-NCAM becomes restricted to a few regions of neural plasticity and regenerating neural tissues. Recently, PSA-NCAM has been shown to be a diagnostic and prognostic marker in adult patients with small cell lung cancer and multiple myeloma, both PSA-NCAM-expressing tumors. In this study, we determined the amount of PSA-NCAM in tumor specimens of nine children with different histologic types and clinical stages of RMS immunohistochemically, using the polysialic acid-specific MAb 735. In seven children, serum levels were investigated by an immunoluminescence assay using the same MAb. Patients with extensive disease showed strong staining of the tumor specimens, whereas patients with limited stages or after chemotherapy had distinctly a lesser amount of PSA-NCAM or almost no staining. Simultaneously, the serum levels were very high (up to 9-fold) in patients with extensive disease, whereas patients with limited disease or after successful therapy had normal serum levels. We conclude that PSA-NCAM expression is high in tumor specimens and serum of patients with advanced stages of RMS and decreases during successful therapy. PSA-NCAM might therefore serve as a marker for diagnosis and monitoring childhood RMS.
Similar content being viewed by others
Main
RMS, the most frequent soft tissue tumor, accounts for about 5% of all pediatric malignancies(1). On the basis of morphologic appearance and clinical features, four major subtypes are differentiated: embryonal, alveolar, botryoid, and pleomorphic RMS. Because cases of botryoid RMS are classified as embryonal, and because pleomorphic RMS are very rare in children, only two basic types-embryonal and alveolar RMS-were considered in this study. Alveolar RMS displays a more aggressive behavior and has a poorer prognosis compared with embryonal RMS(2). Histologically, RMS resemble characteristics seen during the embryonic development of skeletal muscle.
NCAM is known to be involved in skeletal myogenesis and regeneration after muscle denervation or injury(3, 4). Therefore, it is likely that rhabdomyosarcoma cells, in contrast to normal muscle tissue, express high amounts of NCAM. This NCAM has been shown to be the highly sialylated form(5), described before to be reexpressed in tumors such as Wilms' tumor, neuroblastoma, and small cell lung carcinoma(6, 7). Using a MAb highly specific for polysialic acid(8), we investigated tumor specimens of nine patients with RMS of different histologic types and clinical stages and determined their amount of PSA-NCAM. In seven patients, serum levels were tested to evaluate PSA-NCAM as a diagnostic and prognostic marker in childhood RMS.
METHODS
Patients. The clinical data of the patients investigated are summarized in Table 1. The tumor specimens and/or serum samples were obtained from 11 children during routine measures, and further processed as described below. Staging and histologic classification were applied according to criteria set by the International Rhabdomyosarcoma Study Group(9). The project was approved by the local ethical and scientific committees.
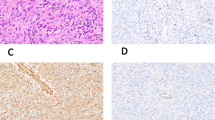
Immunohistochemistry. Cryostat sections (5 μm) of snap frozen tumor specimens were further processed for immunohistochemistry using the APAAP technique according to Cordell(10). Briefly, the slides were fixed in ice-cold acetone for 10 min and air-dried. After preincubation with normal rabbit serum the polysialic acid-specific MAb 735(8) was added for 1 h at room temperature. After washing, the slides were incubated with rabbit anti-mouse immunoglobulin and the alkaline phosphate anti-alkaline phosphate complex twice for 1 h each. Color development was obtained with naphthol AS-biphosphate and New Fuchsin. Positive reaction of the counterstained cells resulted in bright red staining of the cytoplasm. Controls included the omission of primary and/or secondary antibodies, and preincubation of the sections with bacteriophage endoneuraminidase for 2 h at 37°C, which has previously been shown to specifically recognize and degrade polysialic acid(11).
Immunoluminescence assay. Serum samples were thawed and assayed for soluble PSA-NCAM by a chemiluminescent immunoassay developed by the Research Laboratories of Behring Diagnostics, Marburg, Germany(12). In this assay system, the polysialic acid-specific MAb 735(8) is used as a capture antibody. Tris-buffered incubation medium, 200 μL, containing 0.5% Tween 20 (pH 7.0) and 20 μL of sample or standard, was put into tubes coated with MAb 735 and incubated for 1 h at room temperature. After washing, 200 μL of the anti-NCAM MAb BW SCLC-1 conjugated to an acridinium N-acylsulfonamide label were added to each well. After incubation for 1 h at room temperature, the reaction was terminated by an additional washing cycle. The chemiluminescence activity was determined by using the BeriLux Analyzer 250 (Behringwerke AG, Marburg, Germany). The results were expressed as kU/L as previously described(12). All measures were done at least in duplicate for internal control.
RESULTS
As shown in Table 1, all RMS specimens but one showed reactivity with MAb 735, regardless of alveolar or embryonal nature. Patients with extensive disease (nos. 1-6) and the patient with pulmonary metastasis(no. 11) showed strong staining of tumor cells, whereas patients with limited disease (nos. 7-9) had a distinctly lesser amount, or almost no staining. After preincubation with endoneuraminidase, previously positive cells were negative for staining with MAb 735, but remained positive with the NCAM-specific MAb UJ13A(13).
In the serum, the highest PSA-NCAM levels were found in children with stage IV disease (566.1 and 332.1 kU/L), whereas children with stage III and stage II disease had clearly lower serum levels (169.9, 113.4, 106.7, and 78.2 kU/L). One patient with a stage I paratesticular RMS had normal serum levels at diagnosis (25.2 kU/L).
Four children were available for follow-up studies. As shown in Figure 1, the serum concentrations of PSA-NCAM decreased during successful therapy. After reaching normal levels, values remained normal during follow-up. After chemotherapeutic treatment, previously positive tumors became completely negative for PSA-NCAM in immunohistochemical study, as shown in Figure 2.
DISCUSSION
In contrast to other tumors, especially carcinomas in adults, RMS usually respond well to chemotherapeutic agents and radiation. As a consequence, it is not imperative to completely resect an RMS primarily, endangering vital structures. Rather, chemotherapy will reduce the tumor's size and enhance the chance of complete resection at a later operation. Therefore, particularly for these tumors, specific markers are desirable that reflect the viable tumor mass and allow monitoring the response of the tumor cells to chemotherapy or radiation. However, reliable diagnostic and prognostic markers currently are not available for childhood RMS.
RMS cells are known to express the highly sialylated form of the NCAM(5). PSA-NCAM forms are transiently expressed in many tissues during embryogenesis and become restricted to areas of permanent neural plasticity in the adult brain(14). The polysialic acid moiety has been shown to modulate the adhesive functions of NCAM and other adhesion molecules and to be relevant for an enhanced invasive and metastatic potential of tumor cells(15). High levels of PSA-NCAM have been previously shown to be associated with a poor prognosis in adult patients with small cell lung cancer(16). Likewise in our study, the expression of PSA-NCAM was high in advanced stages (stages III or IV) and the pulmonary metastasis, although the lower stages (stages I and II) had considerably less PSA-NCAM. Chemotherapy dramatically removed all PSA-NCAM-positive cells, but expression was high in the pulmonary metastasis that occurred 3 y after treatment of the primary tumor. Because PSA-NCAM was present in both tumor types, embryonal and alveolar, it obviously does not differentiate between them.
NCAM isoforms occur not only membrane-bound but also as soluble molecules detectable in serum, cerebrospinal fluid(17), and amniotic fluid(18). Recently, it has been shown that serum PSA-NCAM is elevated in adult patients with small cell lung cancer(19) and multiple myeloma(20) proving it as a reliable serum marker for these tumors. The mechanism by which NCAM forms appear in the serum is not known. However, the fact that there seems to be a relationship between the serum levels and the estimated amount of PSA-NCAM-positive tumor cells may indicate that there is a constant release of this molecule from the cell surface. This would imply that serum levels of PSA-NCAM, rather than indicating a defined tumor stage or prognosis, reflect the total amount of positive tumor cells, which in turn represent the viable tumor mass. Serum levels decreased during successful therapy and remained low in patients during remission. This indicates that soluble PSA-NCAM may be a useful marker for monitoring therapy in RMS patients.
Abbreviations
- RMS:
-
rhabdomyosarcoma
- NCAM:
-
neural cell adhesion molecule
- PSA:
-
polysialylated
References
Harms D 1995 Soft tissue sarcomas in the Kiel pediatric tumor registry. In: Harms D, Schmidt D (eds) Soft Tissue Tumors. Springer Verlag, Berlin, pp 31–45
Harms D 1995 Alveolar rhabdomyosarcoma: a prognostically unfavorable rhabdomyosarcoma type and its necessary distinction from embryonal rhabdomyosarcoma. In: Harms D, Schmidt D (eds) Soft Tissue Tumors. Springer Verlag, Berlin, pp 273–296
Knudsen KA 1990 Cell adhesion molecules in myogenesis. Curr Opin Cell Biol 2: 902–906
Covault J, Merlie JP, Goridis C, Sanes JR 1986 Molecular forms of N-CAM and its RNA in developing and denervated skeletal muscle. J Cell Biol 102: 731–739
Patel K, Culverwell A, Rossell RJ, Kemshead JT, Phimister E 1993 Vase mini-exon usage by NCAM is not restricted to tumours of neuroectodermal origin. Int J Cancer 54: 772–777
Roth J, Zuber C, Wagner P, Taatjes DJ, Weisgerber C, Heitz PU, Rajewsky K, Bitter-Suermann D 1988 Reexpression of poly(sialic acid) units of the neural cell adhesion molecule in Wilms tumor. Proc Natl Acad Sci USA 85: 2999–3003
Moolenaar CEC, Muller EJ, Schol DJ, Figdor CG, Bock E, Bitter-Suermann D, Michalides RJAM 1990 Expression of neural cell adhesion molecule related sialo-glycoprotein in small cell lung cancer and neuroblastoma cell lines H69 and CHP-212. Cancer Res 50: 1102–1106
Frosch M, Gorgen I, Boulnois GJ, Timmis KN, Bitter-Suermann D 1985 NZB mouse system for production of monoclonal antibodies to weak bacterial antigens: isolation of an IgG antibody to the polysaccharide capsules of Escherichia coli K1 and group B meningococci. Proc Natl Acad Sci USA 82: 1194–1198
Crist WM, Gehan EA, Ragab AH, Dickman PS, Donaldson SS, Fryer C, Hammond D, Hays DM, Herrmann J, Heyn R, Morris Jones P, Lawrence W, Newton W, Ortega J, Raney RB, Ruymann FB, Teft M, Weber B, Wiener E, Wharam M, Vietti TJ, Maurer HM 1995 The third Intergroup Rhabdomyosarcoma Study. J Clin Oncol 13: 610–630
Cordell JL, Falini B, Erber WN, Ghosh AK, Abdulaziz Z, Mac Donald S, Pulford KAF, Stein H, Mason DY 1984 Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase. J Histochem Cytochem 32: 219–229
Gerady-Schahn R, Bethe A, Brennecke T, Mühlenhoff M, Eckhardt M, Ziesing S, Lottspeich F, Frosch M 1995 Molecular cloning and functional expression of bacteriophage PKIE-encoded endoneuraminidase Endo NE. Mol Microbiol 16: 441–450
Takamatsu K, Auerbach B, Gerardy-Schahn R, Eckhardt M, Jaques G, Madry N 1994 Characterization of tumor-associated neural cell adhesion molecule in human serum. Cancer Res 54: 2598–2603
Patel K, Rossell RJ, Bourne S, Moore SE, Walsh FS, Kemshead JT 1989 Monoclonal antibody UJ13A recognizes the neural cell adhesion molecule (NCAM). Int J Cancer 44: 1062–1068
Rougon G 1993 Structure, metabolism and cell biology of polysialic acids. Eur J Cell Biol 61: 197–207
Yang P, Yin X, Rutishauser U 1992 Intercellular space is affected by the polysialic acid content of NCAM. J Cell Biol 116: 1487–1496
Ledermann JA, Pasini F, Olabiran Y, Pelosi G 1994 Detection of the neural cell adhesion molecule (NCAM) in serum of patients with small-cell lung cancer (SCLC) with “limited” or“extensive” disease, and bone-marrow infiltration. Int J Cancer Suppl 8: 49–52
Jørgensen OS, Bock E 1975 Synaptic plasma membrane antigen D2 measured in human cerebrospinal fluid by rocket-line immunoelectrophoresis. Determination in psychiatric and neurological patients. Scand J Immunol 4: 25–30
Ibsen S, Berezin V, Nørgaard-Pedersen B, Bock E 1983 Quantification of the D2-glycoprotein in amniotic fluid and serum from pregnancies with fetal neural tube defects. J Neurochem 41: 363–366
Jaques G, Auerbach B, Pritsch M, Wolf M, Madry N, Havemann K 1993 Evaluation of serum neural cell adhesion molecule as a new tumor marker in small cell lung cancer. Cancer 72: 418–425
Kaiser U, Jaques G, Havemann K, Auerbach B 1994 Serum NCAM: a potential new prognostic marker for multiple myeloma. Blood 83: 871–873
Author information
Authors and Affiliations
Additional information
Supported by Deutsche Forschungsgemeinschaft, Grant GL 173/2-1 (S.G.), and by Boehringer Mannheim Research Fund (R.G.-S.).
Rights and permissions
About this article
Cite this article
Glüer, S., Schelp, C., Von Schweinitz, D. et al. Polysialylated Neural Cell Adhesion Molecule in Childhood Rhabdomyosarcoma. Pediatr Res 43, 145–147 (1998). https://doi.org/10.1203/00006450-199801000-00022
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199801000-00022
This article is cited by
-
A cancer-unique glycan: de-N-acetyl polysialic acid (dPSA) linked to cell surface nucleolin depends on re-expression of the fetal polysialyltransferase ST8SIA2 gene
Journal of Experimental & Clinical Cancer Research (2021)
-
Physical biology of the cancer cell glycocalyx
Nature Physics (2018)
-
Intrabodies against the Polysialyltransferases ST8SiaII and ST8SiaIV inhibit Polysialylation of NCAM in rhabdomyosarcoma tumor cells
BMC Biotechnology (2017)
-
Absence of polysialylated NCAM is an unfavorable prognostic phenotype for advanced stage neuroblastoma
BMC Cancer (2009)
-
Polysialic acid overexpression in malignant astrocytomas
Acta Neurochirurgica (2009)