Abstract
We have previously shown that mild hypothermia applied after hypoxia-ischemia in newborn piglets and rats reduces brain injury evaluated 3-7 d after the insult. The aim of the present study was to assess the neuroprotective efficacy of hypothermia with respect to short-(neuropathology) and long-term (neuropathology and sensorimotor function) outcome after hypoxiaischemia in 7-d-old rats. One hundred fourteen animals from 13 litters survived either 1 or 6 wk after a hypoxic-ischemic insult. The animals were randomized to either 1) normothermic recovery for the whole 1- or 6-wk period or 2) cooling to a rectal temperature of 32.0 °C for the first 6 h followed by normothermic recovery with the dam. Hypothermia offered a uniform protection of 27, 35, 28, and 25% in cerebral cortex, hippocampus, basal ganglia, and thalamus, respectively, in the 1-wk survivors (n = 32). The corresponding values for the 6-wk survivors(n = 61) were 22, 28, 37, and 35%. There was a significant correlation between sensorimotor performance and infarct volume (r = 0.66; p < 0.001). However, the sensorimotor function was not significantly improved by hypothermia if all animals were included, but in female pups the total functional score was higher in the hypothermia group(150 ± 35 versus 100 ± 34, p < 0.0007) which corresponded to a marked (51%) reduction of the neuropathology score in this subgroup. This is the first neonatal study to show a long-term histopathologic protection of the brain after posthypoxic hypothermia.
Similar content being viewed by others
Main
In the clinical setting of stroke, traumatic brain injury, or acute perinatal asphyxia, treatment can start only after the period of the primary insult. Prevention of the cascade of damaging processes in the reperfusion/reoxygenation phase is essential to reduce the mortality and improve the neurologic outcome of survivors. The concept of hypothermia as a treatment against the development of brain injury is not new. Both old and recent clinical studies have indicated that moderate hypothermia effectively limits the progress of cerebral ischemic injury and traumatic brain injury(1–3).
There is overwhelming experimental evidence that intraischemic hypothermia offers long lasting protection [for review see Mahler and Hachinski(4) and Ginsberg et al.(5)]. Duration, magnitude, and onset of hypothermia after the insult are important factors that influence the protective effect of lowering the body or brain temperature. Animal studies of adult brain ischemia have shown contradictory results with respect to long-term effects on neuroprotection; some succeeded(6–8) and others failed to show permanent protection with postischemic moderate hypothermia as the sole treatment(9). Previous studies in immature animals have shown that post hypoxic-ischemic hypothermia protects in the short-term perspective(10–15), but evidence is lacking with regard to long-term efficacy.
We have chosen to study the effects of hypothermia in a model of transient focal HI in the 7-d-old rat(16), which has brain maturation that corresponds to the near term baby(17, 18).
The main aims of the study were to determine whether moderate hypothermia offered both short and long lasting neuroprotection at the histologic and functional level(19). In addition, our purpose was to correlate sensorimotor testing with neuropathologic measures of outcome and finally to evaluate the different methods of quantifying brain injury.
METHODS
Study design. Animal experiments were approved by the ethical committee of Göteborg University (no. 36-96). A total of 126 inbred Wistar Furth (Møllegaard, Denmark) rat pups from 13 litters of both sexes (65 male, 61 female) were used. Of the 126 pups 94 were treated, 20 were controls, and 12 were “thermometer” rats. Within each litter, pups were paired for body weight, and within each pair randomized to either hypo-or normothermia. Pairs were randomized to short: 1 wk (1/3 of litter, n= 32), or long: 6 wk (2/3 of litter, n = 62) survival or to long-term survival without HI in the 20 juvenile controls. Operation order was balanced between each survival group. Long-term survivors and the juvenile controls were tested with respect to sensorimotor function. One normothermic rat (long-term survival group) died during recovery.
Operative procedures. At postnatal d 7 (P7) 94 pups were exposed to HI as follows. The pups were anesthetized with halothane (3.5% for induction and 1.5% for maintenance) in a mixture of nitrous oxide and oxygen(1:1). The left common carotid artery was dissected and cut between double ligatures of prolene sutures (6-0). The duration of anesthesia was <10 min. After the surgical procedure, the wounds were infiltrated with a local anesthetic. The pups were left to recover with the dam for 1 h. Then the litter was placed in a chamber perfused with humidified air (36.0 °C) for preheating for 30 min, and thereafter a gas mixture (7.70 ± 0.01% oxygen in nitrogen, 3 L/min) for 70 min.
Temperature measurements. Calibrated (<0.1 °C deviation) temperature probes (0.4-mm outer diameter thermocouple microprobe type IT-21, Physitemp, Clifton, NJ) were inserted 0.5 cm rectally and taped on to the tail in 2 animals out of the 20 exposed to hypoxia in the same chamber. As previously shown using the same model, rectal temperature is representative of the brain temperature(14). At the end of the hypoxic insult one probe animal and those randomized to normothermia treatment were placed in the normothermic (rectal temperature 37.0 °C) chamber for 6 h. The other probe animal and those randomized to hypothermia treatment were placed in the hypothermic chamber (target rectal temperature 32.0 °C) for 6 h. Rectal temperature together with chamber air temperature was continuously recorded and stored for the hypoxic period and the following 6 h (Lab-VIEW, version 2.5.2 National Instruments, Austin, TX) (Fig. 1).
Mean rectal temperature recordings in the 12“thermometer” P7 rats during preheating and 70 min of hypoxia followed by 360 min of normo- or hypothermia (mean ± SD) and corresponding air temperature in the different chambers. All animals were exposed to hypoxia at 36 °C air temperature in the same chamber.
After 6 h of either normothermic or hypothermic recovery, the probe animals were decapitated, and the blood glucose was analyzed (BM-test-Glycemine 1-44, Boehringer Mannheim). The HI pups were returned to their dams after a total period of separation of 7 h 40 min. They were reared at 22 °C environmental temperature with a light:dark cycle of 12:12 h and food and water ad libitum. On P28 the pups were weaned, and three to five animals (mixed sex) were reared until P49 in individual boxes.
Sensorimotor tests. The last days before testing, the rats were handled by the examiner. The tests were carried out during the first hours of the light portion of the light:dark cycle. Each rat participated 15-20 min per trial. Twenty animals not subjected to ligation or hypoxia served as juvenile controls. The rats were tested at P42 (postural reflex/limb placing and foot-fault test) (for illustrations, see Refs.19–23) and P49 (swing and paw-preference test) (for illustrations see Refs.24 and 25). In the foot-fault test(19, 20) the rat was placed on a horizontal grid floor. A foot-fault was defined as when the animal misplaced a fore- or hindlimb and the paw fell through the grid bars. Number of foot-faults was noted for 2 min. Only the left-right difference in foot-faults was used for the statistical evaluation to eliminate the influence of the extent of activity in different rats. In the postural reflex and limb placing test [modified after previous publications(19, 21–23)] the rat was held by the examiner and the fore- and hindlimb placement after different sensory stimuli was noted. Score 0, immediate and correct paw placing; score 1, delayed and/or incomplete correction; and score 2, no placing. Side differences were noted for each rat. 1) Postural reflex and visual limb placing was tested by lowering the rat toward a table. Normal rats extend the forelimbs toward the table surface, whereas rats with brain damage flex the forelimb contralateral to the damaged hemisphere. 2) Then the rat was placed on the table, held by the tail, and firm lateral pressure was applied behind the shoulder of the rat until the forelimbs slid. A reduced resistance to this lateral force toward the right (contralateral) side was recorded as abnormal. 3) Forelimb sensory input was tested with the rat's forelimbs touching a table edge. 4) Forelimb placement was tested when the rat was facing the edge of the table. A normal rat placed both forepaws on the table top, whereas a rat with a brain lesion failed to correctly place the contralateral paw. 5) Both fore- and hindlimb placement was tested when the rat was held by the examiner and laterally moved toward the edge of the table. 6) When placed on the table the rat was gently pushed laterally toward the edge of the table. A control rat tends to grip on to the edge, whereas an injured rat may drop the forelimb contralateral to the injured hemisphere. 7) As 6) above but the rat was pushed from behind. The swing test [modified after Borlongan et al.(24)] was performed in a glass box with the animal held by its tail and elevated 5 cm above the ground. A swing was defined as the animal turning its head more than 10 ° to either side of the vertical axis. The direction and frequency of the swing was recorded twice for 30 s, and the mean of the two periods was calculated. In the paw-preference test[modified after Boksa et al.(25)] a small block (13-mm diameter, 6 mm thick) was attached with tape between the eyes and the snout of the rat. The order of paw preference (right versus left) and latencies to forelimb contact, to removal of the block, and to grooming were noted for each rat (maximum, 5 min). Each rat was given one trial. All sensorimotor test results were ranked individually from the best to the worst, and then the four ranks for each rat were added to give a total fuctional rank. This rank was correlated to the different structural evaluations. The results of the four separate tests were correlated to each of the four brain regions evaluated by neuropathology, to determine whether we had specific protection in function by any area.
Neuropathology and gross morphology score. On P14 (1 wk of recovery) 32 rats were anesthetized with 0.2 mg/g of body weight methohexital intraperitoneally, and the brains were perfused (transcardiac) with 5 mL of isotonic NaCI and fixed with 5% phosphate-buffered formaldehyde (6-8 mL/min for 5 min). On P49 (6 wk of recovery) 81 rats were anesthetized and perfused(35 mL/min for 5 min) as above. The brains were dissected and kept in 5% formaldehyde at 4 °C until further processing.
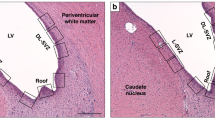
All brains were photographed from the dorsal view to do gross morphology scoring [modified after Yager et al.(26)](scores are defined in Table 1) and to make area and diameter measurements. Coronal blocks cut through the brain were embedded in paraffin, subserially sectioned at 5 μm, and stained with H&E. Ten sections at 1-mm intervals (3.8 mm anterior to 5.2 mm posterior to the bregma) were examined per brain for volume measurement and morphologic scoring (Fig. 2). The extent of the damage was graded from 0.0 to 4.0 with 0.5 intervals for each of the four regions; the cortex, hippocampus, basal ganglia, and thalamus as previously described(14). The pathologic scores for these areas are defined in Table 2(14).
The area of viable tissue in the left and right hemisphere was measured in 10 (1 mm apart) consecutive coronal H&E stained sections, and the infarct volume was calculated by integration (expressed as 100% - volume of intact left hemisphere as percent of the right hemisphere). The line in the figure shows the left hemisphere tissue area of the third rostral section at the striatal level.
Volume measurement. Infarct volume in H&E sections was measured with an image analyzing system consisting of a Nikon Optiphot-2 microscope, a video camera (CCD72, Dage-MTI, Michigan City, IN), a digitizing unit attached to the video camera, and a Macintosh Quadra 900 computer equipped with a video card. The soft ware program Voltum (LabVIEW Run-Time 2.2.1, National Instruments, Austin, TX) was used to measure the area of viable tissue in the left and right hemisphere and calculating the volume of brain damage by integration of the 10 areas over 1-mm intervals (expressed as 100% volume of intact left hemisphere as% of the right hemisphere,Fig. 2). The examiner was blinded to the mode of treatment.
Statistics. The χ2 analysis was used for results in the limb placing test. Mann-Whitney U test was used for differences in neuropathology, gross morphology, volume, area, diameter, body weight, and functional test with continuos data. A Spearman rank correlation test was used for evaluating the correlation between different structural and functional measures. Descriptive data are given as mean ± SD. A p value less than 0.05 was regarded as significant (*p < 0.05,**p < 0.01, ***p < 0.001; NS, p > 0.05).
RESULTS
Body weight, temperature, and blood glucose. The mean body weights in the normothermic, hypothermic, and control groups did not differ significantly at any postnatal age. There was no difference in blood glucose at the end of normo- or hypothermia (5 ± 1 mmol/L and 5 ± 1 mmol/L, respectively, mean ± SD). None of the juvenile control rats had an eyelid ptosis, but 19% of P49 survivors had left eyelid ptosis due to the operative procedure, with no difference between normo- and hypothermic groups.Figure 1 shows recordings of rectal temperature in the 12“thermometer” rats during preheating and 70 min of hypoxia followed by either 360 min normo- or hypothermia (mean ± SD) and the corresponding air temperature in the different chambers.
Evaluation of brain damage. Posthypoxic hypothermia significantly reduced the overall brain damage evaluated with gross morphology in both the P14 and P49 survival groups (Fig. 3). Brain damage evaluated by volume measurement was reduced in hypothermic animals after both short-term (volume deficit 49 ± 24% in normothermia and 34± 26% in hypothermia rats, mean ± SD; NS) and long-term (volume deficit 53 ± 32% in normothermia and 36 ± 25% in hypothermia rats, mean ± SD; p < 0.05) survival. The extent of neuropathology damage score evaluated after short- or long-term survival for each of the four regions; the cortex, hippocampus, basal ganglia, and thalamus are shown in Figure 4,A and B. In the group evaluated at P14, the damage was reduced by approximately 30% in all regions, but the differences were significant only for the hippocampus. In the long-term survival group (P49) the protection was significant for the cerebral cortex, hippocampus, and thalamus. Protection (percent damage reduction in hypothermia compared with normothermia brains) calculated from the different evaluation results in shown in Table 3 with significant reductions noted. In Figure 5 the distribution of animals with different mean neuropathology scores is shown for the normo- and hypothermia group. As the distribution was similar at P14 and P49, the results are pooled. The figure shows that hypothermia was protective against all degrees of damage.
(A and B) The mean pathology score obtained for the left hemisphere based on H&E-stained sections in the normothermic and hypothermic group evaluated P14 (A) and P49(B). The extent of the damage was graded from 0.0 to 4.0 with 0.5 intervals for each of the four regions; the cortex, hippocampus, basal ganglia, and thalamus, at 10 coronal sections distributed throughout the brain(mean ± SD, *p < 0.05, **p < 0.01).
The correlation coefficients between neuropathology and the different structural measures of brain damage at P14 and P49 (gross morphology, infarct volume, area, and diameter) are listed in Table 4.
Figure 6 shows the correlation between the mean neuropathology score and the gross morphology score at both P14 and P49(r = 0.91 and 0.97, respectively). In animals less damaged (mean neuropathology score 0.5-2.0), the gross morphology underestimated the damage as seen by neuropathology.
Functional tests. Functional outcome (total rank of the four tests) was plotted against infarct volume on the ligated side (Fig. 7). There was a significant correlation between total functional rank and infarct volume (r = 0.66, p < 0.0001) for hypo- and normothermia rats. Control rat results are plotted in the figure to show the normal range of variation. When examining each individual test, there was no significant difference between animals being hypothermic for the first 6 h after the insult and those being normothermic; however, they were both different from controls (Table 5). There was no correlation between the extent of any regional injury and the four individual sensorimotor tests.
In female rats the correlation between the total functional rank and infarct volume was 0.77 (Fig. 8), and the mean value of the total functional rank in the hypothermic group (150 ± 35) was significantly different from the normothermic group (100 ± 34). The corresponding mean neuropathology scores for the long-term survival female rats (for all four regions) were 1.4 ± 0.97 for the hypothermic group and 2.8 ± 1.2 for the normothermic group (p < 0.002).
DISCUSSION
This is the first study to show that moderate hypothermia during the initial 6 h of reperfusion offers not only short-term but also long-term protection against the development of brain damage in a neonatal animal model. We also show that functional testing of rats at 7 wk of age correlates with neuropathology. In recent short-term (3-7 d) survival studies with newborn rats or pigs, all showed less damage in the groups made hypothermic for the first 3-12 h after the insult(10, 11, 13–15).
In adult animals, conflicting results exist concerning long-term protection by hypothermia. Dietrich et al.(9) concluded that the benefit of posthypoxic hypothermia might be to increase the therapeutic time window, allowing other therapeutic strategies in the postinsult period. In adult male rats, they found that posthypoxic hypothermic neuroprotection was significant after 3 d of survival, detectable after 7 d, and disappeared after 2 mo; hence they concluded hypothermia merely postponed the damage.
It has been suggested that posthypoxic hypothermia is protective against only moderate and not severe damage according to a recent study in newborn rats(27) and pigs,(11) which is also supported by a clinical study of head trauma(3), demonstrating that patients with a coma score of 3-4 at entry had no improvement at 6 mo follow-up by 24 h of mild (3 °C) hypothermia. In our present study we find that posthypoxic hypothermia equally well protects both mild, moderate, and severely damaged individuals (Fig. 5). This agrees with the result of a previous study with fewer animals and shorter(only 3 h) cooling applied(14), and still the full range of damage was protected. From published data one can speculate that it is in the more mature brain, which develops posthypoxic seizures, that severe brain injury is less protected. Because hypothermia did not reduce the occurrence of seizures in these studies(11, 28), it has been suggested that as much as 72 h of cooling is necessary to abolish seizures(28).
Animal studies usually lack behavioral/neurologic measures of severity of damage. Hence, we were interested in determining whether different functional tests were sensitive enough to correlate with total or regional neuropathology. The results only partially confirm this: an overall correlation between degree of global damage and the total functional rank was obtained. Only in the subgroup of female rats was there a significant correlation between functional improvement and mean neuropathology score (Fig. 8). However, there was no correspondence of individual test result to the neuropathology in different brain regions.
There are several possible explanations for the lack of sensitivity. Immature individuals are capable of different degrees of compensatory adaptation in addition to the extent of structural injury(20, 29). Function might be primarily regulated or taken over by subcortical undamaged structures(30). It has also been shown that environmental factors affect the functional long-term outcome in adult rats subjected to focal ischemia without influencing the structural damage(31). It is possible that a variety of functional tasks including also spatial performance and learning tests as an adjunct to sensorimotor testing would be more sensitive to observe long-lasting functional differences between treatment groups and controls(32, 33).
In the long-term survivors there was a significantly better protection of hypothermia in females than males. Our study was, however, not designed to examine the effect of gender. We had the same total number of males and females in the 13 litters used, but each litter often had an unequal number of males and females. Reanalyzing a previous study(14), there was no difference in the degree of damage between males and females. In this animal model there is a well known and substantial litter dependence on the degree of damage, and therefore further studies are needed before any general conclusions can be drawn with regard to sex.
Neuropathology is the only end point in most experimental studies on neuroprotection. Perfusion fixation and preparations of histologic sections are time-consuming, and their evaluation requires highly specialized investigators. In this study we found an excellent agreement between computerized volume measurements (performed partly by technicians) and semi-quantitative neuropathologic scoring performed by an experienced neuropathologist (Table 4). Furthermore, a nine-step gross morphology score was introduced, extended, and modified from that used by Yager et al.(26), and a close agreement was found to the more time-consuming neuropathologic assessments. As expected, the gross morphology score was less likely to detect smaller degree of damage, which can be seen only microscopically. However, the gross morphology score appears to be an acceptable alternative to be used as a screening evaluation of brain injury in neuroprotective studies, provided that regional information is not requested. Infarct volume measurements showed excellent agreement with neuropathology (Table 4) also at lower degrees of damage. The disadvantage is that this method also requires the time-consuming preparation of histologic sections; however, less training is needed for reliable evaluation.
Conclusion. We have shown that a reduction of 5 °C body temperature lasting 6 h and starting after an hypoxic-ischemic insult offers a significant short-term (1 wk) as well as long-term (6 wk) neuroprotection in the neonatal rat. Survival to 7 wk for a rat corresponds to an age well into puberty(34), hence protection after 6 wk represents long-term protection. The protection is uniform in all areas of the brain, and both severe and moderate to mild insults are reduced by the hypothermic intervention. A total functional score correlated well with neuropathology, but the functional score was not significantly improved by hypothermia. In the long-term surviving female subgroup, however, hypothermia provided a marked reduction of brain damage, which was associated with a significant improvement also of the functional score. Finally, a modified nine-step easy to do gross morphology score correlated well to more sophisticated analysis using quantitative measures of infarct volume or semiquantitative morphologic assessments.
Abbreviations
- HI:
-
hypoxia-ischemia
- H&E:
-
hematoxylin and eosin
- P:
-
postnatal day
References
Westin B, Nyberg R, Miller JA, Wedenberg E 1962 Hypothermia and transfusion with oxygenated blood in the treatment of asphyxia neonatorum. Acta Paediatr Scand Suppl 51: 1–80.
Metz C, Holzschuh M, Bein T, Woertgen C, Frey A, Frey I, Taeger K, Brawanski A 1996 Moderate hypothermia in patients with severe head injury: cerebral and extracerebral effects. J Neurosurg 85: 533–541.
Marion DW, Penrod LE, Kelsey SF, Obrist WD, Kochanek PM, Palmer AM, Wisniewski SR, DeKosky ST 1997 Treatment of traumatic brain injury with moderate hypothermia. N Engl J Med 336: 540–546.
Mahler J, Hachinski V 1993 Hypothermia as a potential treatment for cerebral ischemia. Cerebrovasc Brain Metab Rev 5: 277–300.
Ginsberg MD, Sternau LL, Globus MY-T, Dietrich WD, Busto R 1992 Therapeutic modulation of brain temperature: relevance to ischemic brain injury. Cerebrovasc Brain Metab Rev 4: 189–225.
Colbourne F, Corbett D 1994 Delayed and prolonged post-ischaemic hypothermia is neuroprotective in the gerbil. Brain Res 654: 265–272.
Colbourne F, Corbett D 1995 Delayed postischaemic hypothermia: a six month survival study using behavioral and histological assessment of neuroprotection. J Neurosci 15: 7250–7260.
Coimbra C, Drake M, Boris-Möller F, Wieloch T 1996 Long-lasting neuroprotective effect of postischemic hypothermia and treatment with an anti-inflammatory/antipyretic drug-evidence for chronic encephalopathic processes following ischemia. Stroke 27: 1578–1585.
Dietrich WD, Busto R, Alonso O, Globus MY-T, Ginsberg MD 1993 Intraischemic but not postischemic brain hypothermia protects chronically following global forebrain ischemia in rats. J Cereb Blood Flow Metab 13: 541–549.
Amess PN, Penrice J, Cady EB, Lorek A, Wylezinska M, Cooper CE, Dsouza P, Tyszczuk L, Thoresen M, Edwards AD, Wyatt JS, Reynolds EOR 1997 Mild hypothermia after severe transient hypoxia ischemia reduces the delayed rise in cerebral lactate in the newborn piglet. Pediatr Res 41: 803–808.
Haaland K, Løberg EM, Steen PA, Thoresen M 1997 Posthypoxic hypothermia in newborn piglets. Pediatr Res 41: 505–512.
Laptook AR, Corbett RJT, Sterett R, Burns DK, Garcia D, Tollefsbol G 1997 Modest hypothermia provides partial neuroprotection when used for immediate resuscitation after brain ischemia. Pediatr Res 42: 17–24.
Thoresen M, Penrice U, Lorek A, Cady EB, Wylezinska M, Kirkbride V, Cooper CE, Brown GC, Edwards AD, Wyatt JS, Reynolds EOR 1995 Mild hypothermia after severe transient hypoxia-ischemia ameliorates delayed cerebral energy failure in the newborn piglet. Pediatr Res 37: 667–670.
Thoresen M, Bågenholm R, Løberg EM, Apricena F, Kjellmer I 1996 Posthypoxic cooling of neonatal rats provides protection against brain injury. Arch Dis Child 74: F3–9.
Thoresen M, Wyatt J Keeping a cool head, posthypoxic hypothermia-an old idea revisited. Acta Paediatr Scand 86: 1029–1033.
Rice JE, Vannucci RC, Brierley JB 1981 The influence of immaturity on hypoxicischemic brain damage in the rat. Ann Neurol 9: 131–141.
Romijn HJ, Hofman MA, Gramsbergen A 1991 At what age is the developing cerebral cortex of the rat comparable to that of the full-term newborn human baby?. Early Hum Dev 26: 61–67.
Hagberg H, Bona E, Gilland E, Puka-Sundvall M 1997 Hypoxia-ischemia model in the 7-day-old rat: possibilities and shortcomings. Acta Paediatr Suppl 442: 85–88.
Bona E, Johansson BB, Hagberg H 1997 Sensorimotor function and neuropathology five to six weeks after hypoxia-ischemia in seven-day-old rats. Pediatr Res 42: 678–683.
Barth TM, Stanfield BB 1990 The recovery of forelimb-placing behavior in rats with neonatal unilateral cortical damage involves the remaining hemisphere. J Neurosci 10: 3449–3459.
Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H 1986 Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke 17: 472–476.
De Ryck M, Van Reempts J, Borgers M, Wauquier A, Janssen AJ 1989 Photochemical stroke model: flunarizine prevents sensorimotor deficits after neocortical infarcts in rats. Stroke 20: 1383–1390.
Ohlsson A-L, Johansson BB 1995 Environment influences functional outcome of cerebral infarction in rats. Stroke 26: 644–649.
Borlongan CV, Randall TS, Cahill DW, Sanberg PR 1995 Asymmetrical motor behavior in rats with unilateral striatal excitotoxic lesions as revealed by the elevated body swing test. Brain Res 676: 231–234.
Boksa P, Krishnamurthy A, Brooks W 1995 Effects of a period of asphyxia during birth on spatial learning in the rat. Pediatr Res 37: 489–496.
Yager JY, Heitjan DF, Towfighi J, Vannucci RC 1992 Effect of insulin-induced and fasting hypoglycemia on perinatal hypoxic-ischemic brain damage. Pediatr Res 31: 138–142.
Trescher WH, Ishiwa S, Johnston MV 1997 Brief post-hypoxic-ischemic hypothermia markedly delays neonatal brain injury. Brain Dev 19: 326–338.
Gunn AJ, Gunn TR, deHaan HH, Williams CE, Gluckman PD 1997 Dramatic neuronal rescue with prolonged selective heed cooling after ischemia in fetal lambs. J Clin Invest 99: 248–56.
Castro AJ 1977 Limb preferences after lesions of the cerebral hemisphere in adult and neonatal rats. Physiol Behav 18: 605–608.
Pazos AJ, Orezzoli SL, McCabe PM, Dietrich WD, Green EJ 1995 Recovery of vibrissae-dependent behavioral responses following barrelfield damage is not dependent upon the remaining somatosensory cortical tissue. Brain Res 689: 224–232.
Johansson BB 1996 Functional outcome in rats transferred to an enriched environment 15 days after focal brain ischemia. Stroke 27: 324–326.
Markgraf CG, Green EJ, Hurwitz BE, Morikawa E, Dietrich WD, McCabe PM, Ginsberg MD, Schneiderman N 1992 Sensorimotor and cognitive consequences of middle cerebral artery occlusion in rats. Brain Res 575: 238–246.
Young RSK, Kolnich J, Woods CL, Yagel SK 1986 Behavioral performance of rats following neonatal hypoxia-ischemia. Stroke 17: 1313–1316.
Nakano M, Mizuno T, Gotoh S 1990 Accumulation of cardiac lipofuscin in mammals: correlation between sexual maturation and the first appearance of lipofuscin. Mech Ageing Dev 52: 93–106.
Acknowledgements
The authors thank Fabio Apricena for excellent help with the experiments.
Author information
Authors and Affiliations
Additional information
Supported by the Swedish Medical Research Council Grant 09455, the Sven Jerring foundation, the 1987 Foundation for Stroke reserch, the Åke Wiberg Foundation, the Åhlén Foundation, the Magnus Bergwall Foundation, the Konung Gustaf V's 80 års Foundation, the Frimurare Barnhus Foundation, the First May Flower Annual Campaign, the Linnéa and Josef Carlsson Foundation, the Göteborg Medical Society, Fonden för studerandet av läkarvetenskapen vid Sahlgrenska sjukhuset, the Medical Faculty of Göteborg, University of Göteborg, the Swedish Society for Medical Research, the Laerdal Foundation for Acute Medicine, the Norwegian Research Council, and the Norwegian SIDS Society. M.T. was supported as a guest professor by the Swedish Medical Research Council (11938).
Rights and permissions
About this article
Cite this article
Bona, E., Hagberg, H., Løberg, E. et al. Protective Effects of Moderate Hypothermia after Neonatal Hypoxia-Ischemia: Short- and Long-Term Outcome. Pediatr Res 43, 738–745 (1998). https://doi.org/10.1203/00006450-199806000-00005
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199806000-00005
This article is cited by
-
Hypothermia combined with extracellular vesicles from clonally expanded immortalized mesenchymal stromal cells improves neurodevelopmental impairment in neonatal hypoxic-ischemic brain injury
Journal of Neuroinflammation (2023)
-
Brain organoids for hypoxic-ischemic studies: from bench to bedside
Cellular and Molecular Life Sciences (2023)
-
Azithromycin reduces inflammation-amplified hypoxic–ischemic brain injury in neonatal rats
Pediatric Research (2022)
-
Inter-alpha Inhibitor Proteins Ameliorate Brain Injury and Improve Behavioral Outcomes in a Sex-Dependent Manner After Exposure to Neonatal Hypoxia Ischemia in Newborn and Young Adult Rats
Neurotherapeutics (2022)
-
Variability and sex-dependence of hypothermic neuroprotection in a rat model of neonatal hypoxic–ischaemic brain injury: a single laboratory meta-analysis
Scientific Reports (2020)