Abstract
The effect of creatine (Cr) on the response of the respiratory center to anoxia was analyzed at different postnatal stages in a brainstem slice preparation of mice. Spontaneous rhythmic activity was recorded from hypoglossal rootlets (XII) and from identified neurons within the preBötzinger complex using the whole cell patch clamp technique. The hypoxic response was evaluated in slices from animals (n = 46), which received normal nutrition (controls, n = 16), from litters of animals fed with Cr (2 g/kg/day; nutrition group, n = 8), or after incubating slices for 3 h in Cr (200 μM) (incubation group, n = 22). ATP was measured in slices from controls and Cr-incubated slices which underwent 30-min anoxia. In neonatal animals (P0-5), amplitudes of hypoglossal bursts increased initially during anoxia by 14% in controls and by 41% in Cr-supplemented animals when compared with preanoxic values. Hypoglossal burst duration increased by 3% in controls, but by 18% in the Cr-nutrition group. In brainstem slices, the initial increase of amplitudes changed from 14%(controls) to 59% (Cr incubation) and prolongation of bursts from 3%(controls) to 37% (Cr incubation) compared with preanoxic values. In juvenile controls (P6-13), burst amplitude and duration increased by 12 and 14% during early anoxia when referred to preanoxic values. In slices from Cr-pretreated animals, increases of 48% (amplitude) and 21% (burst duration) occurred. The ATP levels remained constant during a 30-min anoxic period in the Cr-pretreated group compared with a decrease of 44% in slices from controls. Our data suggest that Cr can ameliorate hypoxic energy failure. Further studies will examine the neuroprotective potential in humans.
Similar content being viewed by others
Main
Several pathophysiologic mechanisms are responsible for hypoxia-induced neuronal injury: energy depletion causing activation of K+ conductances(e.g. KATP channels), blockade of ATPase-dependent ion pumps and modulation of second messenger systems(1–3), and finally excessive activation of glutamate receptors leading to a massive influx of calcium(4–8). This leads ultimately to break-down of ionic gradients (Na+, K+, and Ca2+) across plasma membranes and a disturbance of synaptic transmission(9–11). These hypoxia-induced processes seem to be triggered by a fall in intracellular ATP levels, activation of K+ conductances, and glutamate excitotoxicity(12–14). Maintenance of ATP levels is therefore of fundamental importance for protection of brain tissue from hypoxic insult. With decreasing oxygen levels, ATP synthesis relies increasingly on PCr. Previous reports have shown that exogenously applied Cr results in enhanced PCr levels(15–16). In this study we have examined the influence of exogenously applied Cr on the hypoxic response of the spontaneously active respiratory network.
The medullary respiratory network is the most active structure in the brain. It responds biphasically to hypoxia(17–20). Initially the frequency and intensity (measured by burst duration and amplitude) of the respiratory motor output are enhanced (augmentation), which results in increased ventilation to improve oxygen supply. This compensation is particularly important, but energy consuming. Augmentation is more pronounced in adult animals, indicating that they are considerably more oxygen-dependent than neonates. After this short“arousal” response, the second phase of hypoxic depression is characterized by a decreased frequency and intensity of rhythmic respiratory activity. Neonates maintain depressed rhythmic activity for longer periods, whereas mature animals reversibly cease respiratory activity (protective hypoxic apnea) when hypoxia persists. After restoring the oxygen supply, within 8-20 min the depression is followed by a recovery of respiratory rhythmic activity.
The hypoxic response and its maturational changes are maintained after complete isolation of the respiratory center in an in vitro brainstem slice preparation of mice(21). This provides an ideal situation in which to study the direct effects of changes in energy supply on the respiratory center and their relation to the hypoxic response. It is postulated that decreasing levels of ATP and PCr affect the degree of the hypoxic response(11). We therefore studied the hypoxic response of the isolated respiratory network after Cr supplementation. Under pathologic conditions, changes of the PCr level may be an important mechanism for contributing to the hypoxic response.
It is well established that recurrent apneic states in most preterm infants are characterized by the absence of a pronounced hypoxic augmentation(22) and that recurrent apneas are associated with long-term morbidity. Exogenously applied Cr may therefore be a therapeutic treatment to enhance central respiratory drive in premature infants.
METHODS
Preparation. Female and male mice (n = 30) of different postnatal stages (P0-P5 = neonatal animals, P6-P14 = juvenile animals) were deeply anesthetized with ether and decapitated at the C4 level. All animal experiments have been approved by the Institutional Review Board of the University of Göttingen, FRG.
For in vitro experiments, technical details of the transverse brainstem slice preparations have been described elsewhere(21). The brainstem was isolated in ice-cold aCSF and secured in a vibratome with its rostral end tilted at an angle of 20° to the plane of the razor blade. Slices 150 μm thick were sectioned serially until reaching the rostral boundary of the so-called preBÖTc, a region which is critical for the generation of respiratory rhythm(23). The transverse plane containing the preBÖTc is recognized by cytoarchitectonic landmarks, such as the disappearance of the facial nucleus, the appearance of the inferior olive and the XII. The adjacent transverse slice was cut with a thickness of 650-700 μm caudal to this rostral boundary (Fig. 1), immediately transferred into a recording chamber, and submerged under a stream of aCSF (flow rate, 10 mL/min, 29°C, continuously gassed with carbogen (95% O2 and 5% CO2). The aCSF contained (in mM): 128 NaCl, 3 KCl, 1.5 CaCl2, 1 MgSO4, 24 NaHCO3, 0.5 NaH2PO4, and 30 D-glucose equilibrated with carbogen at 27°C to pH 7.4. The potassium concentration in the bath was routinely raised to 8 mM over a period of 30 min to obtain regular rhythmic activity in the XIIs.
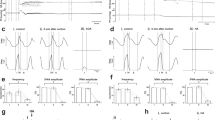
(A) Schematic illustration of a transverse brainstem slice from mice containing the preBÖTc, nucleus ambiguus(NA), nucleus tractus solitarius (NTS), inferior olive(IO), spinal trigeminal nucleus (Sp5), hypoglossal motor nucleus (XII), and hypoglossal rootlet (XII).(B) XII (activity was extracellularly recorded and integrated(XIIi, upper trace). Intracellular activity was recorded in a preBÖTc (pBc) neuron (lower trace).
Anoxia was induced over a period of 30 min by gassing the aCSF with 95% N2 and 5% CO2. After 30-min anoxia, gassing with 95% O2 restored normal O2 supply, and the hypoglossal activity was recovered. The recovery was measured routinely 10 min after restoring O2 supply. Previous oxygen measurements have revealed that slices were well supplied with O2 under such control conditions and became anoxic when they were superfused with aCSF gassed with N2 95%(21).
Respiratory rhythmic activity was analyzed before, during, and after anoxia for controls with normal nutrition (n = 16) in group 1. Group 2(n = 8) preparations came from offsprings of mothers who were fed with Cr (creatine monohydrate, Sigma Chemical Co., Deisenhofen, Germany) 2 g/kg/body weight 10 d before delivery. In group 3 (n = 22) brain slices were Cr-incubated for 3 h by exchanging the superfusate with aCSF containing Cr 200 μM [according to Whittingham and Lipton(9)] before measurements were made.
Anoxia-induced changes are given in percentage change from control conditions for each single experiment during anoxic augmentation, anoxic depression (after 20 min), and 10 min after the end of anoxia (recovery). Percentage change from control conditions before anoxia for untreated and treated slices were analyzed for each single experiment. Significances were determined by the t test.
Neurophysiologic analysis. Respiratory output activity was recorded with a suction electrode from the ends of the XII and integrated by an electronic filter (Paynter filter = 20-30 ms) (Fig. 1B). Simultaneous patch clamp recordings in the whole cell configuration were obtained from single neurons within the preBötzinger complex (Fig. 1B). This area was identified by anatomical landmarks clearly recognizable under a dissection microscope (Zeiss). Somatic recordings were distinguished from axonal recordings by the shape of action potentials and presence of synaptic activity. Patch electrodes manufactured from filamented borosilicate glass (Clarke GC 150 F) were filled with a solution containing in mM: 120 D-gluconic acid, 1 CaCl2, 1 NaCl, 10 HEPES, 5 mM 1,2-bis(2-aminopheonxy)ethane-N,N,N′,N′-tetraacetic acid (tetrapotassium salt), 1 MgCl2, 0.5 NaATP (pH 7.3-7.4). Two amplifiers were used: NPI SEC-10L and Axopatch 200 (Axon Instruments). As respiratory neurons are large, space clamp conditions were not always ideal as seen in the escape of action potential discharge. Data were stored on video tape (VR-100, Instrutech, Inc.) and analyzed on- and offline.
Biochemical analysis. ATP and PCr measurements were performed in 650-μm brainstem slices including the preBÖTc obtained from neonatal animals using enzymatic tests and bioluminescence. Slices of untreated controls (n = 10) and Cr-treated slices (n = 10) were homogenized immediately by ultrasound in 250 μL of 8% perchloric acid and centrifuged for 15 min at 12 000 rpm after preparation, after 3-h incubation (controls n = 10, Cr-treated n = 10), and after 3-h incubation plus 30-min anoxia (controls n = 10, Cr-treated n = 10). The supernatant was neutralized by addition of KHCO3 and recentrifuged. In single slices, ATP was measured using the bioluminescence assay kit HS II of Boehringer Mannheim, Germany. Bioluminescence was detected by the AutoLumat LB 953, EG&G Berthold, Hannover, Germany. Technical details have been described elsewhere(24–26). Protein measurement of slices were performed to relate ATP levels to protein content using the Bradford(27) test.
Enzymatic analyses of ATP and PCr were carried out according to Lamprecht et al.(28). In 100 μL of the sample and an additional 300-μL test solution containing 100 mM triethanolamine, 0.5 mM NADP, 7 mM MgCl2, and 0.3 mM ADP, the enzymatic reaction was started with 0.5 μL of hexokinase, and ATP was measured using a photometer with a light absorption at 365 nm.
RESULTS
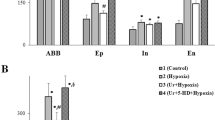
Biochemical results. Biochemical analysis of neonatal tissue(P2) indicated that ATP levels do not differ significantly after Cr nutrition or 3-h incubation in Cr, when compared with controls. In contrast, after 30-min anoxia there was a significant (p < 0.05) decrease of 44% in the ATP level in control slices compared with a stable ATP level in Cr-treated slices (Fig. 2). In juvenile slices ATP levels fell by 28% after 30-min anoxia, against only 10% in the Cr-treated slices. ATP was 9.7 ± 1.4 μmol/g of wet weight) in the Cr-treated group compared with 9.4 ± 0.1 μmol/g in controls (not significantly different). After 30-min anoxia, the ATP concentration was still 9.0 ± 0.1 μmol/g in Cr-incubated slices compared with only 5.1 ± 0.2μmol/g in controls (p = 0.04).
Electrophysiologic results . Hypoglossal activity. Initial augmentation to anoxia was seen as increased amplitude of rhythmic XII burst activity (Fig. 3,A and B). Another characteristic feature, the tonic activation of hypoglossal motoneurons, was reflected by a steady upward deflection in integrated XII activity (arrow in Fig. 3,B and D). In the presence of exogenously applied Cr, there was a reduction of the anoxic depression of the amplitude of rhythmic XII burst discharges and an increase in the tonic activation of the XII motor output (Fig. 3,B and D).
(A and B) The effect of Cr on anoxia recorded extracellularly from hypoglossal nerve rootlets in neonatal animals. (A) Untreated control slice. (B) Response to anoxia for Cr treated slice. In untreated control there is only a small increase in amplitudes during augmentation compared with Cr-treated animal(horizontal arrow in (B). There is not only an increase of amplitudes but also an increase in tonic activation of hypoglossal activity(vertical arrow), indicating an stronger response to anoxia in neonatal as well as juvenile animals treated with Cr. (C and D) The effect of Cr on anoxia recorded extracellularly from hypoglossal nerve rootlets in juvenile animals. (C) Untreated juvenile slice. (D) Cr-treated juvenile slice. Amplitude and tonic activation are elevated in Cr-treated slice compared with the control recording. In both slices there is a complete depression of hypoglossal activity.
Hypoxic responses of slices from litters of animals fed with Cr and slices after Cr-incubation were not significantly different (Fig. 4). Both ways of Cr treatment not only enhanced the amplitude, but also the duration of the augmentation period in neonatal and juvenile slices as compared with neonatal (Fig. 5A) and juvenile control slices (Fig. 5B). In Cr-treated neonatal slices and slices from Cr-fed litters, anoxic augmentation of amplitudes and burst durations of hypoglossal discharges were significantly (p = 0.01) larger than in untreated neonatal slices (Fig. 4). Only burst frequency of hypoglossal discharges remained unaffected. Recovery from anoxia occurred in a similar way.
Effect of Cr during anoxic augmentation and recovery on integrated hypoglossal burst duration, frequency, and amplitude for neonatal P0-5 and juvenile slices P6-13. (A) During augmentation a significant effect was seen in integrated hypoglossal activity(iXII) amplitudes in both Cr groups in neontal as well as in juvenile slices. In both neonatal Cr groups there was a significant increase in burst duration. Differences in iXII frequency are not significant.(B) During recovery 10 min after anoxia, hypoglossal activity was significantly affected in iXII amplitudes and frequency in the juvenile Cr group, reaching nearly pre-anoxic values faster than in untreated controls. Differences in neonatal animals are not significant (n.s.).
Anoxic augmentation for hypoglossal amplitudes for neonatal controls (n = 8) and Cr-treated slices (n = 12)(A), as well as for juvenile untreated controls (n = 7) and Cr treated slices (n = 8) (B). The amplitudes are elevated and the duration of the augmentation period is enhanced. Amplitudes were given in percentage change from preanoxic control.
In Cr-treated juvenile slices and slices from Cr-fed litters, augmentation of the amplitudes of hypoglossal burst discharges was significantly (p = 0.002) increased in comparison with untreated slices. Anoxic changes of hypoglossal burst duration and frequency did not change significantly. Recovery, as measured 10 min after restoring oxygen supply, occurred faster in both groups of Cr-treated juvenile slices. Amplitude of hypoglossal burst were still depressed in untreated slices, whereas in both groups of Cr-treated slices the depression had returned to an almost normal level (Fig. 4). In addition the frequency of hypoglossal bursts had already recovered to control levels in the Cr groups, whereas respiratory frequency was still significantly enhanced in untreated controls. There were no significant changes of burst duration in control and in Cr-treated animals (Fig. 4).
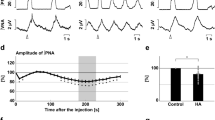
Respiratory neurones. To examine whether the effects recorded in the XII motor output reflect similar changes in the neurones of the respiratory network, we recorded synaptic drive currents from the soma of respiratory neurones in the preBÖTc. Most of the intracellular recordings were not sufficiently stable, and seal resistances became more leaky after the 4 h necessary to examine the changes in single cells induced by Cr incubation. We compared therefore intracellular recordings from respiratory neurons in neonatal control slices (n = 3) and after Cr treatment (n= 5) before and during anoxia. For measurements in the Cr incubation group, we used a new cell recording.
The effect of Cr on the hypoxic response of single respiratory neurones of neonatal slices is shown in Fig. 6. Such measurements showed that there is an increase of the synaptic drive currents in respiratory neurons during the initial augmentation period (n = 15). Synaptic drive currents increased by 14% ± 6 in control cells, whereas in Cr-treated neonatal preparations currents increased by 29% ± 5(p = 0.04). Such synaptic drive often induced firing of action potentials. Rhythmic activity remained for a longer time during hypoxic depression in Cr-pretreated neonatal slices (Fig. 6), whereas inspiratory drive currents decreased in untreated slices (see Fig. 6). In juvenile control slices synaptic drive currents increased during anoxic augmentation by 22% ± 1 (p = 0.003) in Cr-treated juvenile slices (n = 3) and only by 11 ± 4% in untreated slices (n = 4). Also a persistent outward current developed during hypoxic augmentation that was smaller in the Cr-treated group as compared with controls (Fig. 6).
Original recordings from respiratory neurons within the preBÖTc (pBc) and from the hypoglossal nerve rootlets from a untreated neonatal control (left) and a Cr-treated animal(right) during anoxia. Upper panel: control recording before anoxia. Middle panel: improved augmentation characterized by an increase of burst duration, and amplitudes of hypoglossal bursts in treated compared with untreated slices. The synaptic drive currents in the preBÖTc neuron increase remarkable. Lower panel: hypoglossal activity is suppressed in the untreated slice whereas amplitude is still elevated in the Cr-treated slice during depression 10 min after starting anoxia. The synaptic drive potential of a respiratory neuron is nearly complete suppressed during depression, but nearly stable in the Cr-treated neonatal animal. Note there is no difference in recovery between treated and untreated neonatal animals. The cellular recording is given in detail at the left and right side (gray bars).
DISCUSSION
The integrity of brain activity is maintained as long as there is a sufficient supply of energy in form of ATP. Cr increases PCr, which is an important reserve for direct ATP synthesis in muscle and brain(11, 29).
Using biochemical methods, we have demonstrated that, under normoxic conditions, exogenously applied Cr has no significant effect on the ATP content of neonatal isolated brainstem slices containing the preBÖTc. PCr levels increase by about 20-30% (our unpublished data). However, after 30 min of anoxia, ATP was significantly depleted in the control group, whereas ATP levels were maintained in neonatal slices pretreated directly or indirectly with Cr. These findings confirm that, in neonates, endogenous ATP synthesis alone is not sufficient to stabilize ATP levels for 30 min of anoxia in the neonatal brainstem. More importantly we demonstrated that the anoxia-induced fall in ATP levels can be buffered by exogenously applied Cr. Cr applied either by feeding pregnant mother animals or by incubating slices for 3 h, stabilizes ATP levels in the neonates by forming PCr, which is known to be the major pool for ATP synthesis under anoxic conditions(11, 29). ATP buffering by exogenously applied Cr enhances and stabilizes hypoglossal activity during anoxia.
Hypoglossal neurons depolarize faster under hypoxia than do cortical neurons(30, 31). The hypoglossal neurons are especially vulnerable and depolarize after a shorter delay. Nucleus XII neurons partly recover poorly from anoxia, which is well tolerated by neocortical neurons(32). These data suggest that the hypoglossal system is highly energy dependent during anoxia and is a suitable system to study the effects of exogenously applied Cr.
ATP is needed to maintain transmembrane ionic homeostasis and to maintain voltage- and ligand-controlled membrane currents. Experimental block of ATP-sensitive potassium channels with glibenclamide enhances depolarization of hypoglossal neurons of about 25%(3). Excessive excitation may then activate voltage-regulated channels leading to massive calcium ion fluxes and destruction of neurons. Recently it has been demonstrated in different types of energy failure that compensatory rescue mechanisms in neonatal neuronal cells are underpowered(33). The immature NMDA-type glutamate receptor and channel complex contains subunits that allow the channel to be opened more easily and for a longer period resulting in a selective vulnerabilty to NMDA receptor overstimulation during hypoxia(33). The resulting failure in synaptic transmission could be prevented by increasing the supply to the tissue with high energy phosphates. Preincubation of neocortical cells with Cr had a pronounced protective effect on excitatory synaptic transmission(34).
The potency of exogenously applied Cr to delay a decrease of ATP levels was demonstrated by an altered response of the transverse slice preparation to anoxia. Augmentation in juvenile control animals is characterized by an increased amplitude of rhythmic hypoglossal activity, which in the presence of exogenous Cr is slightly enhanced, suggesting that ATP and PCr pools of juvenile animals are more stable than those of neonates. In contrast in neonatal control animals of the age group P0-5, augmentation is characterized by a significant increased frequency and a slight increase in amplitude of rhythmic hypoglossal activity. In the presence of Cr, hypoxic amplitude modulation is significantly enhanced. This indicates that hypoxic fall of ATP occurs quickly in neonatal animals, resulting in reduced augmentation of activity. Cr supplementation thus improves the energy supply from the very beginning of hypoxia beyond control conditions. This is consistent with the finding that PCr was completely depleted during anoxia in immature, but only reduced by 40-50% in mature, rat brains(35, 36). These maturational changes might be explained by the finding that Cr kinase activity increases during postnatal development(37, 38).
We suggest that the generation of a significant augmentation depends on the presence of a sufficiently large energy pool including PCr. This assumption might have important clinical implications. It is known that most premature infants suffering from recurrent apnea exhibit no or only a weak hypoxic augmentation of respiration(39). In these cases, exogenously applied Cr could be a potential therapy for protection against hypoxic depression.
Using in vivo magnetic resonance-spectroscopy, the first inborn error of Cr synthesis was recently recognized(40). The patient, a 2-y-old boy, showed a developmental delay beginning at the age of 3-6 mo combined with muscular hypotonia and severe extrapyramidal movement disorder. The nucleus pallidus, known to be a highly energy-dependent structure, showed abnormal signals on magnetic resonance imaging. Neurologic symptoms, the developmental delay, and pathologic magnetic resonance imaging signals of the nucleus pallidus normalized or improved after oral substitution with Cr monohydrate. It was demonstrated that supplementation of Cr increases the Cr pool in the brain and that this Cr becomes biologically active(41).
Our data indicate that exogenous application of Cr contributes to protection against acute anoxia by increasing the augmenting response and prolonging survival of cerebral synaptic transmission. Cr may thus be an efficient neuroprotective substance and be considered a potential therapeutic in preterm infants with apnea and children threatened by hypoxic events,e.g. asphyxia during birth.
Abbreviations
- aCSF:
-
artificial cerebrospinal fluid
- Cr:
-
creatine
- PCr:
-
phosphocreatine
- XII:
-
hypoglossal rootlets
- NMDA:
-
N-methyl-D-aspartate
- preBÖTc:
-
preBötzinger complex
References
Ben Ari Y 1989 Effects of glibenclamide, a selective blocker of an ATP-K+ channel, on the anoxic response of hippocampal neurons. Plügers Arch 414( suppl 1): S111–S114
Ben Ari Y 1990 Modulation of ATP sensitive K+ channels: a novel strategy to reduce the deleterious effects of anoxia. Adv Exp Med Biol 268: 481–489
Jiang C, Haddad GG 1991 Effect of anoxia on intracellular and extracellular potassium activity in hypoglossal neurons in vitro. J Neurophysiol 66: 103–111
Choi DW 1988 Glutamate neurotoxicity and disease of the nervous system. Neuron 1: 623–634
Choi DW 1990 Cerebral hypoxia: some new approaches and unanswered questions. J Neurosci 10: 2493–2501
Rothman SM, Olney JW 1986 Glutamate and the pathophysiology of hypoxicischemic brain damage. Ann Neurol 19: 105–111
Rothman SM, Olney JW 1987 Excitotoxicity and the NMDA receptor. TINS 10: 299–302
Lutz PL 1992 Mechanisms for anoxic survival in the vertebrate brain. Annu Rev Physiol 4: 601–618
Whittingham TS, Lipton P 1981 Cerebral synaptic transmission during anoxia is protected by creatine. J Neurochem 37: 1618–1621
Lipton P, Whittingham TS 1982 Reduced ATP Concentration as a basis for synaptic transmission failure during hypoxia in the in vitro guinea-pig hippocampus. J Physiol 325: 51–65
Vannucci RC 1990 Experimental biology of cerebral hypoxic-ischemia: relation to perinatal brain damage. Pediatr Res 27: 317–326
Katayama Y, Kawamata T, Tamura T, Hovada DA, Becker DP, Tsubokawa T 1991 Calcium-dependent glutamate release concomitant with massive potassium flux during cerebral ischemia in vivo. Brain Res 558: 136–140
Martin RL, Lloyd HGE, Cowan AI 1994 The early events of oxygen and glucose deprivation: setting the scene for neuronal death?. TINS 17: 251–257
Takata T, Okada Y 1995 Effects of deprivation of oxygen or glucose on the neural activity in the guinea pig hippocampal slice-intracellular recording study of pyramidal neurons. Brain Res 683: 109–116
Holtzman D, McFarland E, Moreland T, Koutcher J, Kushmerick MJ, Neuringer LJ 1989 Brain creatine phosphate and creatine kinase in mice fed an analogue of creatine. Brain Res 483: 68–77
Balestrino M 1995 Pathophysiology of anoxic depolarization: new findings and a working hypothesis. J Neurosci Methods 59: 99–103
Haddad GG, Mellins RB 1984 Hypoxia and respiratory control in early life. Annu Rev Physiol 46: 629–643
Martin-Body RL, Johnston BM 1988 Central origin of the hypoxic depression of breathing in the newborn. Respir Physiol 71: 25–32
Neubauer JA, Melton JE, Edelman NH 1990 Modulation of respiration during brain hypoxia. J Appl Physiol 68: 441–449
Richter DW, Bischoff A, Anders K, Bellingham M, Windhorst U 1991 Response of the medullary respiratory network of the cat to hypoxia. J Physiol 443: 231–256
Ramirez JM, Quellmalz UJA, Richter DW 1996 Postnatal changes in the mammalian respiratory network as revealed by the transverse brainstem slice of mice. J Physiol 491: 799–811
Upton CJ, Milner AD, Stokes GM 1991 Apnea, bradycardia, and oxygen saturation in preterm infants. Arch Dis Child 66: 381–385
Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL 1991 Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254: 726–729
DeLuca M, McElroy WD 1978 Purification and properties of firefly luciferase. Methods Enzymol 57: 3–15
Lundin A, Hasenson M, Persson J, Pousette A 1986 Estimation of biomass in growing cell lines by adenosine triphosphate assay. Methods Enzymol 133: 27–42
Stanley PE 1986 Extraction of adenosine triphosphate from microbial and somatic cells. Methods Enzymol 133: 14–22
Bradford MM 1976 A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254
Lamprecht W, Stein P, Heinz F, Weisser H 1974 Kreatinphosphat: Methoden der enzymatischen Analyse in Bergmeyer HU, Verlag Chemie Weinheim Band II Seite 1825–1829
Erecinska M, Silver IA 1989 ATP and Brain Function. J Cereb Blood Flow Metab 9: 2–19
Haddad GG, Donnelly DF 1990 O2 Deprivation induces a major depolarization in brain stem neurons in the adult but not in the neonatal rat. J Physiol 429: 411–428
Donnelly DF, Jiang C, Haddad GG 1992 Comparative responses of brain stem and hippocampal neurons to O2 deprivation: in vitro intracellular studies. Am J Physiol 262:L549–L554
O'Reilly JP, Jiang C, Haddad GG 1995 Major differences in response to graded hypoxia between hypoglossal and neocortical neurons. Brain Res 683: 179–18186
Johnston MV 1995 Neurotransmitters and vulnerability of the developing brain. Brain Dev 17: 301–306
Luhmann HJ, Heinemann U 1992 Hypoxia-induced functional alterations in adult rat neocortex. J Neurophysiol 67: 798–811
Tsuji M, Allred E, Jensen F, Holtzman D 1995 Phosphocreatine and ATP regulation in the hypoxic developing rat brain. Dev Brain Res 85: 192–200
Pierard C, Champagnat J, Denavit-Saubie M, Gillet B, Beloeil JC, Guezennec CY, Barrer B, Peres M 1995 Brain stem energy metabolism response to acute hypoxia in anaesthetized rats: a 31P NMR study. NeuroReport 7: 281–285
Norwood WI, Ingall JS, Norwood CR, Fossel ET 1983 Developmental changes of creatine kinase metabolism in rat brain. Am J Physiol 244:C205–C210
Holtzman D, Tsuji M, Wallimann T, Hemmer W 1993 Functional Maturation of creatine kinase in rat brain Dev N. eurosci 15: 261
Ruggins NR 1991 Pathophysiology of apnea in preterm infants. Arch Dis Child 66: 70–73
Stöckler S, Holzbach U, Hanefeld F, Marquardt I. Helms G, Requart M, Hänicke W, Frahm J 1994 Creatine deficiency in the brain: a new, treatable inborn error of metabolism. Pediatr Res 36: 409–413
Frahm J, Bruhn H, Hanefeld F 1995 Proton NMR studies of human brain metabolism. In: Belton PS, Delgadillo I, Gil AM, Webb GA (eds). Proceedings of the 2nd International Conference on Applications of Magnetic Resonance in Food Science. Royal Society of Chemistry, Cambridge, UK, pp 191–205
Author information
Authors and Affiliations
Additional information
Supported by grants from the Sonderforschungsbereich (SFB) 406.
Rights and permissions
About this article
Cite this article
Wilken, B., Ramirez, J., Probst, I. et al. Creatine Protects the Central Respiratory Network of Mammals under Anoxic Conditions. Pediatr Res 43, 8–14 (1998). https://doi.org/10.1203/00006450-199801000-00002
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199801000-00002
This article is cited by
-
Important roles of dietary taurine, creatine, carnosine, anserine and 4-hydroxyproline in human nutrition and health
Amino Acids (2020)
-
Early postnatal exposure to intermittent hypoxia in rodents is proinflammatory, impairs white matter integrity, and alters brain metabolism
Pediatric Research (2017)
-
Creatine kinase in ischemic and inflammatory disorders
Clinical and Translational Medicine (2016)
-
Potential of creatine or phosphocreatine supplementation in cerebrovascular disease and in ischemic heart disease
Amino Acids (2016)
-
Creatine for women: a review of the relationship between creatine and the reproductive cycle and female-specific benefits of creatine therapy
Amino Acids (2016)