Abstract
Preterm newborns have low serum thyroxine (T4) levels compared with late-gestational fetuses. Low thyroid hormone levels are associated with increased severity of neonatal illness and neurodevelopmental dysfunction. We assessed the endocrine and clinical effects of increasing serum T4 levels in preterm newborns with a gestational age <31 wk. Forty newborns were randomized in a double blind protocol: 20 infants received a daily dose of 20 μg/kg l-T4 for 2 wk, whereas 20 control infants received saline. Serum concentrations of T4, triiodothyronine (T3), reverse T3 (rT3), thyroglobulin (TG), and TSH were measured weekly as well as serum levels of GH, prolactin, and IGF-I. After 2 wk, a TSH-releasing hormone (TRH) test was performed. Neonatal illness and outcome was evaluated by noting heart rate, oxygen requirement, duration of ventilation, development of chronic lung disease, oral fluid intake, and weight gain; a Bayley score was done at the corrected age of 7 mo. l-T4 administration induced a marked increase in serum T4 without apparent change in T3 levels, whereas the postnatal decline in serum rT3 was more gradual. l-T4 treatment was associated with a decrease in serum TG and TSH levels. TRH injection induced a definite rise in serum TSH and T3 in controls, but not in L-T4 treated newborns. Neither l-T4 treatment, nor TRH administration appeared to alter circulating levels of prolactin, GH, or IGF-I. In contrast to the pronounced endocrine effects, no clinical effects of l-T4 administration were detected.
Similar content being viewed by others
Main
L-T4 and TBG are detectable in serum of the human conceptus by 8-10 wk of gestation. TBG, and total and free T4 steadily increase until the final weeks of pregnancy. The mean cord concentration of total T4 around 35 wk of gestation is about 130 nmol/L. Postnatally, circulatory T3 is produced in peripheral tissues via monodeiodination of T4. This deiodination process appears to mature early in the third trimester of gestation. After 30 wk, the serum T3 level increases to a mean of about 0.75 nmol/L near term(1–3). As the secretion of TSH increases despite rising free T4 concentrations, the set point for T4-induced negative feedback changes in late gestation(4, 5).
Thyroid hormones have important stimulatory effects on perinatal maturation processes in the nervous system, heart, lung, and gastrointestinal tract(1–10). There is a linear increase in the postnatal prevalence of hypothyroxinemia with decreasing gestational age. These low T4 concentrations are associated with normal or low TSH concentrations(11–13). It is at present uncertain whether these changes result from an appropriate adaptation mechanism or represent true secondary hypothyroidism. Accordingly, it is currently unknown whether hypothyroxinemia of prematurity requires treatment(13–16). However, recent studies suggest a relationship between hypothyroxinemia in the preterm infant and increased mortality rate or later neurodevelopmental delay and emphasize the need for randomized, controlled studies exploring the therapeutic potential of thyroid hormone administration(17–23).
Here we report a study examining the endocrine and clinical effects of high dose L-T4 treatment in preterm newborns.
METHODS
The study was performed in 1992 according to a randomized, placebo-controlled, double-blind design. All inborn infants with a gestational age of 25-30 wk and from whom cord blood was available were eligible for inclusion in this study. Exclusion criteria were major congenital malformations and maternal thyroid disorders.
Infants were randomized to either a therapeutic regimen of 20 μg/kg weight of L-T4 in saline (20 μg/mL), or were given saline. The medication was administered i.v. in a single dose at 1800 h; 1 infant was fed entirely enterally between d 8 and 14 and received L-T4 at the same dose orally during this phase. L-T4 was administered for 2 wk.
Weekly, at 0800 h, blood was sampled for measurement of serum levels of T4, T3, free (unbound) T4, rT3, TSH, TBG, and TG as well as serum concentrations of PRL, GH, and IGF-I, and urine was collected for measurement of urinary iodine content during the first 6 postnatal weeks.
A single polycythemic newborn received a therapeutic standardized, isovolumetric, partial exchange transfusion over 6 h as previously described(24). By coincidence, L-T4 was administered i.v. during this procedure, and this permitted the study of the dynamics of serum T4 and T3 concentrations immediately before and after L-T4 injection in a total of 19 sequential samples obtained with an interval of 20 min.
On d 14, a TRH test was performed, provided the newborn was in a stable clinical condition. TRH (50 μg) was administered i.v.; serum TSH, GH, and PRL were measured before and 30, 60, and 120 min after TRH.
The serum concentrations of T4, T3, free (unbound) T4, rT3, GH (polyclonal antibody), and IGF-I (after acid ethanol extraction) were measured by standard RIA. TSH and PRL serum values were measured by immunoradiometric assay; the lower detection limit for TSH was 0.02 mU/L.
Gestational age was estimated from maternal dates, from ultrasound assessments in early pregnancy or, when this information was not available, from physical and neurologic assessment according to the Ballard score.
Daily, the individual heart rate (mean of 24 hourly determinations), the inspired oxygen concentration (mean of 12 2-h determinations) and the amount of oral feeding (percent of total fluid intake) were noted during the first 3 wk. Body weight was determined weekly throughout the study period.
The duration of total or partial parenteral nutrition, the duration of mechanical ventilation, and the development of chronic lung disease(25) were also assessed. Psychomotor development was assessed at the corrected age of 7 mo using the Bayley mental and psychomotor developmental index(26). It is noteworthy that surfactant was not yet registered for clinical use in Belgium when this study was performed.
Statistical analysis was performed according to a Mann-Whitney test. The biochemical results are presented as medians with quartiles, the clinical observations as mean ± SEM. To determine statistically significant intergroup differences for gender, the use of prenatal steroids and the incidence of cesarean section and hyaline membrane disease, a χ2 test was used.
The Ethical Authority of the Department of Pediatrics, University Hospital Gasthuisberg, and Medical School of the University of Leuven approved the study protocol and agreed to waive the requirement for written informed consent before study inclusion.
RESULTS
Study population. A total of 40 inborn infants were enrolled. There were no significant differences between study groups for gestational age, Apgar scores, birth weight, or sex ratio, for prenatal treatment with corticosteroids and/or TRH, for mode of delivery, or for the incidence of severe respiratory distress syndrome (hyaline membrane disease grade III-IV)(Table 1). Three newborns in each subgroup died within the first weeks; in the control group the cause of death was congenital infection with shock (d 1), respiratory failure (d 10), congenital infection and intracranial bleeding (d 14); in the L-T4-treated group the cause of death was pulmonary hypoplasia (d 1), ventricular septum defect and cardiorespiratory failure (d 15; on autopsy, also horseshoe kidney), respiratory failure and intracranial bleeding (d 5); the data from these six children are not included in the following analysis.
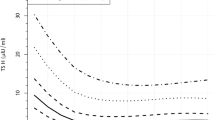
Thyroid axis. The effects of L-T4 administration on the neonatal thyroid axis are depicted in Figure 1. L-T4 treatment results in a marked increase of the serum T4 level without concomitant increase in T3 concentrations. The T4 level on cord blood was 84.9 (65.6-87.5) nmol/L in the treatment group and 77.2 (60.5-98.5) nmol/L in the placebo group (p = NS). During L-T4 treatment, serum T4 levels rose to 131.3 (96.5-184.0)versus 56.6 (48.9-99.1) nmol/L in controls on d 7 (p < 0.001), and to 140.3 (124.8-166.0) versus 75.3 (59.2-95.2) nmol/L, respectively, on d 14 (p < 0.001). One week after discontinuation of L-T4 supplementation, serum T4 declined to a level comparable to that of untreated infants (87.5 (75.9-112.0) versus 88.8(66.9-94.0) nmol/L) (p = ns).
In the weekly measurements, the T3 course is the same for both groups. However, immediately after L-T4 administration, a transient doubling of serum T3 was observed in the single newborn studied during partial exchange transfusion (Fig. 2).
Serum rT3 levels were elevated in cord serum in both study groups[5.2 (4.8-5.8) versus 5.4 (4.3-7.7) nmol/L] (p = ns). The postnatal decline of rT3 is attenuated by L-T4 treatment [3.3(3.0-3.9) versus 1.6 (0.8-2.0) nmol/L on d 7 (p < 0.001) and 2.8 (2.0-2.9) versus 1 (1-1.6) nmol/L on d 14(p < 0.01)], suggesting that the administered L-T4 is, at least partially, converted to rT3. L-T4 administration is associated with a marked decrease in serum TSH concentrations [0.10(0.02-0.30) versus 1.25 (0.68-2.7) mU/L on d 7 (p < 0.001) and 0.08 (0.02-0.10) versus 1.9 (1.5-2.6) mU/L on d 14(p < 0.001)]. One week after discontinuation of therapy, a rise in the serum TSH level was seen at least up to the level of the control group[2.6 (1.4-4.2) versus 1.8 (1.0-2.5) mU/L (p = NS)].
The serum TG concentrations were significantly lower on d 14 in the treated group compared with the control group [24.4 (15.2-37.1) versus 74.7(38.0-95.5) μg/L (p < 0.01)]. After treatment was discontinued the course was similar for both groups. The urine iodine concentrations describe a similar course as the serum T4 levels.
The TRH test was performed on d 14, the last day of supplementation (Fig. 3). Before TRH, TSH values were significantly different (p < 0.001). Controls presented a definite TSH rise at 30, 60, and 120 min, whereas the treated group showed a lower, if any, TSH response (p < 0.001 at 30, 60, and 120 min). In response to TRH stimulation, a marked rise in serum T3 levels is seen in the placebo group, whereas no rise is seen in L-T4-treated newborns [T3 at 120 min 138.5 (94-151) nmol/L in controls versus 80.5 (76-102) nmol/L in L-T4-treated newborns; p < 0.01].
PRL, GH, and IGF-I. The serum PRL, GH, and IGF-I concentrations were at each time comparable in infants treated with L-T4 and in controls. Accordingly, pooled, longitudinal results are presented in Figure 4. By the end of the first postnatal week, serum PRL, GH, and IGF-I concentrations were approximately half of those observed at birth; thereafter, they remained at the same level. TRH failed to provoke a PRL or GH response in control and L-T4-treated infants (data not shown).
Pooled serum concentrations of PRL (A), GH(B), and IGF-I (C) from L-T4-treated and control newborns at birth and at weekly intervals during the first 6 postnatal weeks. At the age of 1 wk, the plasma levels of newborns receiving dopamine for cardiovascular support (▪) were compared with those of newborns who were not receiving dopmine (□). Values are presented as median and quartiles.*p < 0.05, ***p < 0.001 for dopamine effect.
Effect of concomitant dopamine treatment. Seven L-T4-treated and seven control infants were still receiving dopamine infusion on d 7 as part of their standard support, independently of this study. We compared the serum values of T4, T3, TSH (Fig. 5), PRL, GH, and IGF-I (Fig. 4) in the dopamine-treated versus nontreated patients. T4, T3, PRL, and GH results are significantly lower in infants receiving dopamine independently of L-T4 treatment.
Clinical data. L-T4 treatment had no significant effect on heart rate, on inspiratory oxygen concentration, on percentage of enteral fluid intake, or on weight gain (Fig. 6). Duration of mechanical ventilation was also similar (10 ± 3 d with L-T4versus 14 ± 4 d without L-T4 treatment; p = NS) as was the prevalence of chronic lung disease (11/17 surviving infants in each group).
The Bayley score at the corrected age of 7 mo was comparable for both groups, the mental developmental index being 97 (±3) in L-T4 treated and 102 (±1) in control infants, whereas the psychomotor developmental index was 88 (±3) and 93 (±2), respectively(p = NS).
DISCUSSION
This study shows that the low serum T4 and low urinary iodine concentrations characterizing preterm newborns can be increased into the normal range of late-gestational fetuses or term newborns by administering L-T4 in a single daily dose of 20 μg/kg(1–5). Although L-T4 administration was documented to have a profound impact on the neonatal thyroid axis (increased T4 and rT3, decreased TSH and TG), this treatment appeared to have no clinical effects that were readily detectable during the first weeks of postnatal life.
The lack of immediately detectable effect on clinical variables may be related to a preferential conversion of circulating T4 toward rT3 rather than to T3(27). However, the data from the partial exchange transfusion suggest that there may be a brief rise of circulating T3 immediately after T4 administration. Spontaneous and TRH-induced TSH release were suppressed by L-T4 treatment, indicating that the circulating T4 exerts an inhibitory action at the pituitary level, which is independent of circulating T3, but may be mediated by intrapituitary conversion to T3.
In the absence of L-T4 treatment, TRH injection was shown to elicit a brisk TSH response that was accompanied by a surge of circulating T3. These findings suggest that the low T3 syndrome in the preterm newborn includes a hypothalamic component and that-as the peripheral conversion of T4 to T3 is limited-the TRH-induced rise of serum T3 is directly of thyroid origin and mediated by TSH.
In preterm newborns, circulating PRL, GH, and IGF-I were found to decrease promptly during the first postnatal week and to remain stable thereafter. The L-T4 treatment failed to prevent the neonatal fall of serum PRL, GH, or IGF-I, and exogenous TRH did not elicit a PRL or GH response. In contrast, dopamine infusion on d 7 was associated with decreased serum concentrations of PRL and GH, as well as of T4 and T3. These findings are fully consistent with the known inhibitory effect of dopamine infusion on PRL and GH secretion, as well as on circulating T4 and T3 in term newborns(28–30). However, the design of our study does not permit inference of a direct dopamine effect.
The present study characterized the changes that exogenous L-T4 induces in the thyroid axis of the preterm newborn, but failed to identify any clinical benefit or adverse event. Consequently, these data point to the feasibility of L-T4 administration and suggest a L-T4 dose, but do not establish the necessity of this treatment. It is anticipated that the latter issue will have to be resolved by large, multicenter trials with a long follow-up of the children studied.
Abbreviations
- T4:
-
thyroxine
- L-T4:
-
L-thyroxine
- T3:
-
triiodothyronine
- rT3:
-
reverse triiodothyronine
- TG:
-
thyroglobulin
- TBG:
-
thyroid-binding globulin
- TRH:
-
TSH-releasing hormone
- PRL:
-
prolactin
References
Fisher DA, Klein AH 1981 Thyroid development and disorders of thyroid function in the newborn. N Engl J Med 304: 702–712.
Fisher DA, Polk DH 1989 Development of the thyroid. Baillieres Clin Endocrinol Metabol 3: 627–657.
Burrow GN, Fisher DA, Larsen PR 1994 Maternal and fetal thyroid function. N Engl J Med 331: 1072–1078.
Ballabio M, Nicolini U, Jowett T, Ruiz de Elvira MC, Ekins RP, Rodeck CH 1989 Maturation of thyroid function in normal foetuses. Clin Endocrinol 31: 565–571.
Thorpe-Beeston JG, Nicolaides KH, Felton CV, Butler J, McGregor AM 1991 Maturation of the secretion of thyroid hormone and thyroid stimulating hormone in the fetus. N Engl J Med 324: 532–536.
Birk E, Tyndall R, Erickson LC, Rudolph AM, Roberts JM 1992 Effects of thyroid hormone on myocardial beta-receptor responsiveness and function during late gestation. Pediatr Res 31: 468–473.
Lebenthal E, Lee PC 1983 Interaction of determinants in the ontogeny of the gastrointestinal tract: a unified concept. Pediatr Res 17: 19–24.
de Zegher F, Pernasetti F, Vanhole C, Devlieger H, Van den Berghe G, Martial J 1995 The prenatal role of thyroid hormone evidenced by fetomaternal Pit-1 deficiency. J Clin Endocrinol Metab 80: 3127–3130.
Hetzel BS, Dunn JT 1989 The iodine deficiency disorders: their nature and prevention. Annu Rev Nutr 9: 21–38.
Calvo RM, Obregon MJ, Ruiz de Ona C, Escobar del Rey F, Morreale de Escobar G 1990 Congenital hypothyroidism as studied in rats: crucial role of maternal thyroxine but not of 3,5,3′-triiodothyronine in the protection of the fetal brain. J Clin Invest 86: 889–899.
Eggermont E, Vanderschueren-Lodeweyckx M, De Nayer Ph, Smeets E, Vanacker G, Cornette C, Jaeken J, Devlieger H, Eeckels R, Beckers C 1984 The thyroid-system function in preterm infants of postmenstrual ages 31 wk or less: evidence for a “transient lazy thyroid system.”. Helv Paediatr Acta 39: 209–222.
Mercado M, Yu VYH, Francis I, Szymonowicz W, Gold H 1988 Thyroid function in very preterm infants. Early Hum Dev 16: 131–141.
Hadeed AJ, Asay LD, Klein AH, Fisher DA 1981 Significance of transient postnatal hypothyroxinemia in premature infants with and without respiratory distress syndrome. Pediatrics 68: 494–498.
Chowdhry P, Scanlon JW, Auerbach R, Abbassi V 1984 Results of controlled double-blind study of thyroid replacement in very low-birth-weight premature infants with hypothyroxinemia. Pediatrics 73: 301–304.
Amato M, Pasquier S, Carasso A, Von Muralt G 1988 Postnatal thyroxine administration for idiopathic respiratory distress syndrome in preterm infants. Hormone Res 29: 27–30.
Karna P 1991 Developmental follow-up of very low birthweight premature infants with low free thyroxine. Am J Perinatol 8: 288–91.
Schönberger W, Grimm W, Emmrich P, Gempp W 1981 Reduction of mortality rate in premature infants by substitution of thyroid hormones. Eur J Pediatr 135: 245–253.
de Vries LS, Heckmatt JZ, Burrin JM, Dubowitz LMS, Dubowitz V 1986 Low serum thyroxine concentrations and neural maturation in preterm infants. Arch Dis Child 862: 866
Kohelet D, Arbel E, Goldberg M, Arlazzaroff A 1992 Transient neonatal hypothyroxinemia and the auditory brainstem evoked response. Pediatr Res 32: 530–531.
Meijer WJ, Verloove-Vanhorick SP, Brand R, van den Brande JL 1992 Transient hypothyroxinaemia associated with developmental delay in very preterm infants. Arch Dis Child 67: 944–947.
Marsh TD, Freeman D, McKeown RE, Bowyer FP 1993 Increased mortality in neonates with low thyroxine values. J Perinatol 3: 201–204.
Den Ouden AL, Kok JH, Verkerk PH, Brand R, Verloove-Vanhorick SP 1996 The relation between neonatal thyroxine levels and neurodevelopmental outcome at age 5 and 9 years in a national cohort of very pretrem and/or very low birth weight infants. Pediatr Res 39: 142–145.
Reuss ML, Paneth N, Pinto-Martin JA, Lorenz JM, Susser M 1996 The relation of transient hypothyroxinemia in preterm infants to neurologic development at two years of age. N Engl J Med 334: 821–827.
de Zegher F, Devlieger H, Veldhuis JD 1992 Pulsatile and sexually dimorphic secretion of luteinizing hormone in the human infant on the day of birth. Pediatr Res 32: 605–607.
Northway WH, Rosan RC, Porter DY 1967 Pulmonary disease following respirator therapy of hyaline-membrane disease. N Engl J Med 267: 357–374.
Bayley N 1969 Manual for the Bayley Scales of Infant Development. The Psychological Corporation, New York
Van Wassenaer AG, Kok JH, Endert E, Vulsma T, de Vijlder JJM 1993 Thyroxine administration to infants of less than 30 weeks' gestational age does not increase plasma triiodothyronine concentrations. Acta Endocrinol 129: 139–146.
de Zegher F, Van den Berghe G, Devlieger H, Eggermont E, Veldhuis JD 1993 Dopamine inhibits neonatal growth hormone and prolactin hypersecretion. Pediatr Res 34: 642–645.
de Zegher F, Van den Berghe G, Dumoulin M, Gewillig M, Daenen W, Devlieger H 1995 Dopamine suppresses thyroid-stimulating hormone secretion in neonatal hypothyroidism. Acta Pediatr 84: 213–214.
Van den Berghe G, de Zegher F, Lauwers P 1994 Dopamine suppresses pituitary function in infants and children. Crit Care Med 22: 1747–1753.
Acknowledgements
The authors thank the Medical and Nursing Staff of the Neonatal Intensive Care Unit for their cooperation in this study.
Author information
Authors and Affiliations
Additional information
Supported by the Belgian Study Group for Pediatric Endocrinology, the University of Leuven (OT 95/24), and the Belgian Fund for Scientific Research(G-0162-96).
Rights and permissions
About this article
Cite this article
Vanhole, C., Aerssens, P., Naulaers, G. et al. L-Thyroxine Treatment of Preterm Newborns: Clinical and Endocrine Effects. Pediatr Res 42, 87–92 (1997). https://doi.org/10.1203/00006450-199707000-00014
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199707000-00014
This article is cited by
-
Clinical indicators that influence a clinician’s decision to start L-thyroxine treatment in prematurity with transient hypothyroxinemia
Italian Journal of Pediatrics (2023)
-
Effect of levothyroxine supplementation in extremely low birth weight infants with transient hypothyroxinemia of prematurity
Scientific Reports (2022)
-
Effects of oral iodine supplementation in very low birth weight preterm infants for the prevention of thyroid function alterations during the neonatal period: results of a randomised assessor-blinded pilot trial and neurodevelopmental outcomes at 24 months
European Journal of Pediatrics (2022)
-
Incidence and severity of transient hypothyroxinaemia of prematurity associated with survival without composite morbidities in extremely low birth weight infants
Scientific Reports (2019)
-
An explanatory randomised placebo controlled trial of levothyroxine supplementation for babies born <28 weeks’ gestation: results of the TIPIT trial
Trials (2013)