Abstract
This study was undertaken to determine whether simultaneous activation of clotting, fibrinolysis, and kinin-kallikrein is associated with disease severity in preterm infants with neonatal respiratory distress syndrome (RDS), during the first 5 d of life. In the infants with severe RDS, we found activation of clotting, fibrinolysis, and kinin-kallikrein within 6-12 h of birth, indicated by increased thrombin-antithrombin III complex formation[22.5 ng/ml versus 1.4 ng/ml (median values) in the mild/moderate RDS infants, p < 0.001], increased tissue-type plasminogen activator plasma concentrations [5.1 ng/ml versus 2.6 ng/ml (median values) in the mild/moderate RDS infants, p < 0.01], and increased plasma kallikrein activity [198% versus 189% of maximal activated human plasma (median values) in the mild/moderate infants,p < 0.01], respectively. Thrombin generation, tissue-type plasminogen activator release, and kallikrein activity did not change significantly in the severe RDS group throughout the study. In these infants, kallikrein activity was accompanied by lower values of plasma kallikrein inhibitory activity. Activation of clotting, fibrinolysis, and kinin-kallikrein was accompanied with a transient decrease of the neutrophil count and a steady decrease of the platelet count in the severe RDS group. The studied parameters of clotting and fibrinolytic and kinin-kallikrein activation were significantly correlated with continuous measures of RDS severity. We, therefore, suggest that this activation process likely contributes to respiratory insufficiency in neonatal RDS.
Similar content being viewed by others
Main
Increased alveolar-capillary permeability with subsequent leakage of protein in the pulmonary interstitium and the small airways is one of the pathophysiologic hallmarks of the neonatal RDS that develops within only a few hours of birth(1, 2). The degree of this protein leakage is inversely correlated with lung maturity(3). The alveolar-capillary membrane of the more immature lung seems to be more vulnerable to damaging factors such as barotrauma caused by high pressure ventilation(4). Because increased protein leakage has also been described in unventilated lung segments of preterm lambs(5), other factors than barotrauma are thought to contribute to increased alveolar-capillary membrane permeability in neonatal RDS.
Activation of the plasma clotting and fibrinolytic and kinin-kallikrein systems has been described in preterm infants with severe RDS(6–10) and could be one of the contributing factors. Clotting is characterized by thrombin generation(6–8), which occurs in face of low plasma concentrations of antithrombin III (the main inhibitor of thrombin)(7, 8) and results in fibrin formation. Intravascular and intraalveolar fibrin deposits have been found in the lungs of preterm infants, who died from severe RDS(7, 11), despite activation of the fibrinolytic system(6, 7). Therefore, intravascular and intraalveolar fibrinolysis is thought to be insufficient in these infants(6, 12). Activation of the kinin-kallikrein system has been found early in the course of severe RDS and is not influenced by rescue treatment with one dose of natural porcine surfactant(9, 10). In the ARDS, it has been demonstrated that products of the activated clotting, fibrinolytic, and kinin-kallikrein systems are able to increase alveolar-capillary permeability directly or indirectly by sequestration of activated leukocytes and platelets in the lungs(13–15).
If activation of clotting, fibrinolysis, and kinin-kallikrein contributes to increased protein leakage into the lungs, one would expect an association between this activation process and the severity of RDS. Recently, a positive correlation between clotting activation and disease severity has been described in 3-d-old preterm infants with RDS(8). Saugstad et al.(10) described lower prekallikrein levels in preterm infants with severe RDS than in preterm infants with mild/moderate RDS, suggesting more activation of the kinin-kallikrein system in severe RDS. However, it is not known whether simultaneous activation of clotting, fibrinolysis, and kinin-kallikrein is correlated with severity of RDS during the first days of life. We, therefore, measured plasma concentrations of the T-AT III complex (representing clotting activation) and t-PA (representing fibrinolytic activation), PKKI, and kallikrein activity (kinin-kallikrein activation), and total leukocyte, neutrophil, and platelet counts simultaneously in preterm infants with RDS during the first 5 d of life and correlated these parameters with Fio2, PIP, and continuous measures of RDS severity (VE; a/APo2).
METHODS
Patients. Thirty-three preterm infants were studied. These infants were admitted to the neonatal intensive care unit of the Beatrix Childrens Hospital, University Hospital Groningen, between December 1, 1993, and May 1, 1994. Inclusion criteria for study enrollment were: 1) no maternal infection, amnionitis, or prolonged rupture of membranes (>24 h before birth); 2) gestational age between 27 and 33 wk; 3) birth weight appropriate for gestational age; 4) no evidence of infection till 4 d after study completion; and 5) no major congential malformations. All study infants showed clinical and radiologic signs of RDS. These infants were prospectively classified according to the clinical and roentgenologic criteria that were used in the Dutch multicenter study “Nedsurf” for surfactant replacement therapy. Seventeen infants had severe RDS (severe RDS group), which was defined as oxygen requirement of more than 30% for adequate oxygenation, artificial ventilation dependency because of respiratory failure, and roentgenologic features on a chest x-ray consistent with a score 3 or 4 according to Giedion et al.(16). Sixteen infants had mild/moderate RDS(mild/moderate RDS group), which was defined as extra oxygen requirement for adequate oxygenation, artificial ventilation or CPAP dependency because of respiratory failure, and roentgenologic features on a chest x-ray consistent with a score 1 or 2 according to Giedion. The study was approved by the hospital ethical committee, and informed consent was obtained from the parents of all infants.
There were no differences between both RDS groups for prenatal factors that could influence the activation of clotting, fibrinolysis, kinin-kallikrein, and the neutrophil and platelet count (Table 1). The mothers of five infants in the severe and of three infants in the mild/moderate RDS group showed the syndrome of HELLP and/or toxicosis before delivery, which is sometimes accompanied by diminished neutrophil and platelet counts in the newborn infants(17, 18). Neutrophil and platelet counts of these infants could not be distinguished from that of the infants in both groups, whose mothers did not show HELLP and/or toxicosis. The neutrophil and platelet counts of the three mild/moderate RDS infants were higher than those of the five severe RDS infants. The mothers of the infants in both RDS groups did not show other diseases that could influence the number of neutrophils and platelets and the activation of clotting, fibrinolysis, and kinin-kallikrein in their children. Dexamethasone treatment to induce fetal lung maturation was given to the mothers of seven infants in the severe and five infants in the mild/moderate group more than 2 d before delivery. Dexamethasone reduces fibrinolysis(19). Despite maternal dexamethasone treatment, activation of fibrinolysis was not different between the infants antenatally exposed to dexamethasone and the other infants in each group. No other specific drugs that could influence the activation process investigated in this study, such as anticoagulants and cyclooxygenase inhibitors, were given to the mothers. Although we did not observe any statistical significant influence of prenatal factors on the parameters we studied, one should realize that the numbers of infants that were compared were fairly small.
Postnatal characteristics of the severe and mild/moderate RDS group are presented in Table 2. Despite lower Apgar scores and arterial umbilical pH values in the severe RDS infants, no organ failure other than respiratory failure existed in these infants. According to the routine of our unit, serial cranial ultrasonography was performed by the attending pediatric radiologist, who was unaware of disease severity in each RDS infant. Intracranial hemorrhages, if present, were staged according to Levene et al.(20). We did not observe polycythemia(venous hematocrit >0.65) in any infant of either study group throughout the study period.
Patient management. Infants were respiratory supported and/or received extra oxygen to maintain the arterial Po2 between 7.5 and 10.0 kPa and the arterial Pco2 between 5.5 and 6.5 kPa. All severe RDS infants and eight mild/moderate RDS infants required positive pressure ventilation throughout the study period. The remaining of infants with mild/moderate RDS required positive pressure ventilation until the 3rd d of life and supplementary oxygen afterward (n = 4), CPAP throughout the study period (n = 4), or positive pressure ventilation after CPAP during the first 3 d of life (n = 1).
In this study, three infants with mild/moderate RDS received 50 mg/kg bovine surfactant (Alvofact, Boehringer, Ingelheim, Germany), whereas 15 infants with severe RDS were treated with 100 mg/kg surfactant endotracheally according to the criteria of the Nedsurf study. Two infants with severe RDS did not receive surfactant because of suspicion of pulmonary hemorrhage.
According to the local protocol, infants less than 34 wk of gestational age with respiratory failure requiring artificial ventilation or CPAP underwent echocardiographic screening on the 2nd to 4th d of life for a patent ductus arteriosus. A patent ductus arteriosus showing left-to-right shunting was treated with one or two courses (three times 0.2 mg/kg) of i.v. indomethacin(Indocid, Merck Sharpe & Dohme, Haarlem, The Netherlands). Eight infants in each RDS groups received indomethacin.
Eleven infants in the severe RDS group and 13 infants in the mild/moderate RDS group received phototherapy for hyperbilirubinemia. Both RDS groups were not different with regard to the mean serum bilirubin concentration (data not shown). Neither the number of infants who received phototherapy nor the duration of phototherapy was different between the two study groups. Within each study group, platelet counts of the infants who received phototherapy did not differ from those who did not receive phototherapy.
Infants received transfusions of CPP or packed red blood cells with CPP to replace blood taken for routine laboratory tests. The severe RDS group received a higher mean number of transfusions (3 ± 2) throughout the study period than the mild/moderate RDS group (1 ± 1). However, the transfusion volume did not exceed 10% of the calculated blood volume in any 24-h period for each infant in the study. CPP is stored frozen and contains only native inactive plasma proteins, whereas packed red blood cells contain only 10-20% plasma, including plasma proteins, which are slightly activated by the processing procedure(21). Therefore, we do not consider replacement transfusions to influence the findings in the infants of both study groups(6, 21).
Venous and arterial umbilical catheters and peripheral arterial catheters were inserted or removed only after approval of the attending neonatologist. The patency of arterial catheters was maintained by a continuous infusion of 0.9% NaCl solution containing 3 U/ml heparin at a rate of 0.3-0.5 mL/h. Such low dose heparin infusion does not influence clotting in preterm infants(22). As shown in Table 2, all study infants had an arterial umbilical or peripheral arterial catheter, whereas 17 also had a venous umbilical catheter.
Study protocol. Blood samples were taken from an arterial catheter. We were aware of the susceptibility of some parameters to sampling errors and tried to minimize the influence of sampling on them. Blood samples collected from only a free-flowing arterial catheter showing an undamped pressure curve were used for analysis. Furthermore, umbilical catheters were known to be echographically patent. During sampling, the first 3 mL were not used for analysis in this study. Each sample was obtained into a heparinized syringe and then immediately transferred to a plastic tube containing an appropriate anticoagulant to prevent activation of blood before processing of the sample.
Blood samples were taken on the 1st d of life between 6 and 12 h of birth, and on the 2nd, 3rd, 4th, and 5th day of life. The first sample was taken before any medical treatment, including replacement of surfactant, administration of indomethacin or blood products, and phototherapy, had occurred. Each sample was used for the determination of the total leukocyte and neutrophil count, the platelet count, and the activation of clotting (T-AT III complex), fibrinolysis (t-PA), and kinin-kallikrein (kallikrein activity, PKKI).
At each sampling, 0.3 mL of blood was anticoagulated with EDTA (0.01 M) for determination of the total leukocyte count, differential cell counts, and the platelet count; 0.5 mL of blood was anticoagulated with citrate (0.3%) and immediately centrifuged, and the plasma was stored at -20°C until kallikrein activity, PKKI, and plasma concentration of t-PA were determined. Another 0.5 mL of blood was collected in a tube containing hirudin-aprotinin and immediately centrifuged. The plasma of this sample was stored at -20°C until the T-AT III complex concentration was determined.
In each study infant, the Fio2 was recorded, when a blood sample was taken. In study infants, who were artificially ventilated, the PIP was recorded and the VEI and a/APo2 were calculated. The VEI was calculated according to Notter et al.(23) using the following equation: 3800/(DP × F × Paco2), where 3800 is a constant relating to CO2 production (mL × mm Hg/kg× min), DP is the PIP minus the PEEP (cm H2O), F is the ventilatory frequency, and Paco2 is the arterial Pco2 (mm Hg). This index estimates the overall ventilation efficiency of mechanically ventilated infants accounting for the combined effect of different ventilatory pressures, rates, and Pco2 values. The VEI increases as lung function improves. The a/APo2 was calculated by dividing the arterial oxygen tension (Pao2) measured directly from an arterial blood sample by the alveolar oxygen tension (PAo2) calculated using the following equation: Fio2 × (PATM - PH2O) - Paco2, where PATM is the atmospheric pressure (760 mm Hg), PH2O is the partial pressure of water vapor (47 mm Hg), and Paco2 is the arterial carbon dioxide tension(24).
Assays. The platelet and total leukocyte count was determined using a cell counter (Hemolog, Coulter Electronics, London, UK). Platelet counts <50 × 109/L were verified by phase contrast microscopy. The total leukocyte count was determined after correction of the total nucleated cell count for the presence of nucleated red cells (erythroblasts). The leukocytes were differentiated by morphologic classification of 100 cells in blood films, which were stained by the May-Grünwald-Giemsa method. The T-AT III complex concentration in plasma was measured by immobilization of the complex with europium-labeled monoclonal antibodies against the T-AT complex(Est-1, American Diagnostica Inc., Greenwich, CT). The amount of bound europium label was counted by means of time resolved fluorometry (Wallac, Turku, Finland). The t-PA concentration in plasma was determined similarly using europium-labeled monoclonal antibodies against t-PA (Esp-1, American Diagnostica Inc., Greenwich, CT). Kallikrein activity of plasma was measured according to a new method using a colorometric assay (NovaBiochem, Laufelfingen, Switzerland)(25). The kallikrein activity was expressed as a percentage of the activity in maximally activated adult human plasma. PKKI was determined by a colorometric assay (Kabi Diagnostica, Upsala, Sweden) and expressed as the percentage of kallikrein inhibition in normal adult human plasma.
Statistical analysis. Data are presented as mean ± SD or as median with 25th and 75th percentiles as appropriate. The χ2 test with Yates' correction for continuity was used for comparison of nominal data between the severe and mild/moderate RDS group. Comparison of gestational age, birth weight, arterial umbilical pH values, and transfusion numbers between the two groups was carried out using the unpaired t test. For comparison of the 1- and 5-min Apgar scores between the two groups the Mann-Whitney U test was used.
For PIP values, a/APo2, platelet counts, and PKKI values differences between both RDS groups were determined on d 1 (6-12 h) and d 5 by means of the unpaired t test, because these data show a roughly normal distribution. A paired t test was used to compare PIP values, a/Ao2, platelet counts, and PKKI values on d 2, 3, 4, and 5 with the values of these parameters on d 1. For Fio2 and VEI values, total leukocyte and neutrophil counts, plasma T-AT III and t-PA concentrations, and plasma kallikrein activity values, the Mann-Whitney U test and the Wilcoxon signed rank test were used to determine specific differences between and within the two RDS groups, because these data did not show a normal distribution. Adjustment of the significance level for multiple comparisons was made according to the Bonferroni correction as appropriate.
Correlations between the parameters studied (total leukocyte, neutrophil, and platelet counts, T-AT III and t-PA concentrations, kallikrein activity, and PKKI values) and the PIP, Fio2, VEI values, and a/APo2 were determined by calculating Spearman's rank correlation coefficient.
Statistical significance was assumed when the p value was less than 0.05.
RESULTS
Ventilatory requirements. The severe RDS group required more ventilatory support than the mild/moderate group as indicated by higher median Fio2 values (25th to 75th percentiles) on d 1 (0.70 (0.67-0.83)versus 0.37 (0.23-0.43), p < 0.001) and d 5 (0.30(0.27-0.81) versus 0.21 (0.21-0.25), p < 0.01) and higher mean PIP values (± SD) on d 1 (23 ± 3 cm H2O versus 20 ± 3 cm H2O, p < 0.05) and d 5(26 ± 7 cm H2O versus 18 ± 3 cm H2O). Fio2 values decreased significantly in the severe RDS group from d 1 to d 4 (p < 0.05), but not in the mild/moderate RDS group. PIP values did not change in both RDS groups.
Measures of RDS severity. Median VEI values (25th to 75th percentiles) were lower in the ventilated severe RDS infants than in the ventilated mild/moderate RDS infants throughout the study period, being significant on d 1 (0.08 (0.06-0.09) versus 0.10 (0.08-0.15),p < 0.01) and d 5 (0.06 (0.04-0.22) versus 0.27(0.16-0.52), p < 0.05). VEI values did not change in the severe RDS group, but increased significantly in the mild/moderate RDS group between d 1 and 5 (p < 0.05). The mean a/APo2 (±SD) of the severe RDS infants increased significantly from 0.12 ± 0.05 on d 1 to 0.27 ± 0.16 on d 5 (p < 0.05) but was significantly lower than that of the mild/moderate RDS group on d 1 (0.30 ± 0.15,p < 0.001) and d 5 (0.43 ± 0.20, p < 0.05). The a/APo2 of the mild/moderate RDS group did not change significantly during the study period.
Total leukocyte count. The total leukocyte count was lower in the severe RDS group than in the mild/moderate RDS group, being significant on d 5 (p < 0.05). The severe RDS group showed a significant decrease of the median total leukocyte count (25th to 75th percentile) from 5.6 (4.6-7.0) × 109/L on d 1 to 3.8 (1.8-6.1) × 109/L on d 2 (p < 0.05), and an increase afterward to 6.6(4.6-8.4) × 109/L on d 5 (not significant versus d 1). The total leukocyte count of the mild/moderate group increased significantly from 6.9 (5.8-11.6) × 109/L on d 1 to 8.8 (8.0-9.9) × 109/L on d 5 (p < 0.05).
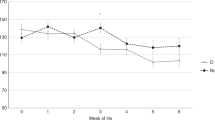
Neutrophil count. On the 1st and 5th d of life, neutrophil counts were significantly lower in the severe than in the mild/moderate RDS group (Fig. 1). In the severe RDS group, the neutrophil count decreased significantly from within 6-12 h of birth to d 4 (p< 0.05) and increased afterward. The neutrophil count did not change in the mild/moderate RDS group.
Neutrophil counts in preterm infants with severe RDS(open boxes) and mild/moderate RDS (gray boxes) during the first 5 d of life. Data are presented as box plots showing the median values (horizontal plane line), ranges of 50% around the median value (boxes), and the 10th and 90th percentile (error bars). *p < 0.05 for severe RDS group compared with mild/moderate RDS group.
Platelet count. The platelet count (mean ± SD) of the severe RDS group decreased significantly from within 6-12 h of birth to d 4(p < 0.05), but that of the mild/moderate RDS group did not change significantly. The two study groups differed with regard to the platelet count being significant on d 5 (p < 0.01)(Fig. 2).
T-AT III complex. On the 1st and 5th d of life, the T-AT III complex concentration of the severe RDS infants was significantly higher than that of the mild/moderate RDS infants (p < 0.001 and p< 0.05, respectively) (Fig. 3). T-AT III complex concentrations did not change significantly in either study group.
Plasma concentrations of T-AT III complex in preterm infants with severe RDS (open boxes) and mild/moderate RDS (gray boxes) during the first 5 d of life. Data are presented as box plots showing the median values (horizontal plane line), ranges of 50% around the median value (boxes), and the 10th and 90th percentile (error bars). *p < 0.05,***p < 0.001 for severe RDS group compared with mild/moderate RDS group.
t-PA. Throughout the study period, the t-PA plasma concentration of the severe RDS group was higher than that of the mild/moderate RDS group, being significant on d 1 and 5 (p < 0.01) (Fig. 4). Neither of the RDS groups showed significant changes in the t-PA plasma concentration during the first 5 d of life.
Plasma concentrations of t-PA in preterm infants with severe RDS (open boxes) and mild/moderate RDS (gray boxes) during the first 5 d of life. Data are presented as box plots showing the median values(horizontal plane line), ranges of 50% around the median value (boxes), and the 10th and 90th percentile (error bars). **p < 0.01 for severe RDS group compared with mild/moderate RDS group.
Kallikrein activity. Both RDS study groups differed significantly on d 1 and 5 (p < 0.01) regarding their plasma kallikrein activity values (Fig. 5) but did not show significant changes in plasma kallikrein activity values throughout the study period.
Plasma kallikrein activity in preterm infants with severe RDS (open boxes) and mild/moderate RDS (gray boxes) during the first 5 d of life. Data are presented as box plots showing the median values(horizontal plane line), ranges of 50% around the median value (boxes), and the 10th and 90th percentile (error bars). **p < 0.01 for severe RDS group compared with mild/moderate RDS group. AHP, activated adult human plasma.
PKKI. PKKI values of the severe RDS infants were significantly lower than those of the mild/moderate RDS infants on d 1 and 5 (p< 0.05) (Fig. 6). PKKI values of both study groups increased slightly but not significantly from the 1st to the 5th d of life.
Correlations. The 5-min Apgar score was positively correlated with the platelet count (ρ = 0.37, p = 0.036), and negatively correlated with the T-AT III complex (ρ = 0.53, p = 0.0031) and the t-PA (ρ = -0.43, p = 0.019) plasma concentration at 6-12 h of birth. The arterial pH value was positively correlated with the platelet count (ρ = 0.58, p = 0.009) and negatively correlated with the plasma concentration of T-AT III complex (ρ = -0.55, p = 0.016) at 6-12 h of birth. Gestational age and birth weight were not significantly correlated with the immediate postnatal values of the studied parameters.
In Table 3, the correlations between the parameters studied (total leukocyte, neutrophil, and platelet counts; T-AT III and t-PA concentrations; kallikrein activity; and PKKI values), the ventilatory support values (PIP and Fio2), and RDS severity measures (VEI and a/APo2) are presented. Mean values of the studied parameters calculated for each infant were not significantly correlated with birth weight and gestational age.
DISCUSSION
Systemic activation of clotting, fibrinolysis, and kinin-kallikrein has been found in preterm infants with severe RDS and is thought to contribute to respiratory failure in these infants(6–10). An association between plasma protein activation and disease severity has been demonstrated for clotting(8) and kinin-kallikrein activation(8, 10) in preterm infants with RDS. In the present study, we have found simultaneous activation of clotting, fibrinolysis, and kinin-kallikrein in 17 preterm infants with severe RDS(severe RDS group) during the first 5 d of life, whereas such activation was almost absent in 16 preterm infants with mild/moderate RDS (mild/moderate group). In the severe RDS group, thrombin generation, t-PA release, and kallikrein activation did not change throughout the study period. Simultaneously, a transient decrease of the neutrophil count and a steady decrease of the platelet count were observed in this RDS group.
In this study, we have found a correlation between continuous measures of RDS severity (VEI and a/APo2) and activation of clotting, fibrinolysis, and kinin-kallikrein. One could argue that activation of these plasma protein systems reflects only the severity of RDS without contributing to it. However, in ARDS(26–29), this activation process is considered not to be a coincidental phenomenon but an important determinant of lung injury. Therefore, we suggest that activation of of clotting, fibrinolysis, and kinin-kallikrein contribute to RDS severity. Plasma levels of products of clotting, fibrinolysis, and kinin-kallikrein might be useful to assess RDS severity in addition to clinical and radiologic scoring systems(16, 30).
In the severe RDS group, increased T-AT III complex plasma concentrations indicate formation of thrombin. T-AT III complex concentrations in the mild/moderate RDS group were in the range of normal adult values(8). Our findings agree with Schmidt et al.(8), who have described thrombin formation in 3-d-old preterm infants with severe RDS. In these infants, thrombin inhibition is impaired because of low AT III plasma concentrations(7, 8). Unopposed formation of thrombin likely increases RDS severity by increase of pulmonary vascular permeability directly or indirectly by conversion of fibrinogen into fibrin(12). Fibrin microthrombi have been found in the pulmonary vascular bed of preterm infants, who died from severe RDS(7, 11). Fibrin thrombi increase alveolar-capillary membrane permeability(31) with subsequent leakage of fibrin and other plasma proteins into the lungs. In oxygen-induced lung injury, intravascular deposition of fibrin precedes formation of fibrin-rich hyaline membranes(32). Increase of plasma proteins in the alveoli results in surfactant inactivation(33, 34).
Increased t-PA plasma concentrations in the severe RDS group agrees with previous findings in severe RDS(6) and indicates activation of the fibrinolytic system. T-PA plasma concentrations in the mild/moderate group were within the range of those in cord blood of normal term newborns(12). In the presence of fibrin, t-PA converts plasminogen into plasmin, that, in turn, causes lysis of fibrin(ogen) with formation of fibrin(ogen) degradation products. Fibrin depositions in the lungs suggests that intravascular and intraalveolar fibrinolysis is insufficient in preterm infants with severe RDS despite increased t-PA concentrations. This might be explained by several factors including 1) decreased plasma levels of plasminogen in face of normal or increased plasma levels of antiplasmin and t-PA inhibitors(35), 2) resistance to t-PA-induced lysis of small fibrin clots such as those seen in the pulmonary vascular bed of infants with severe RDS(36), and 3) depressed t-PA concentrations(37) and limited availability of plasma plasminogen(38) in the face of normal or increased clotting activity in the small airways of preterm infants with RDS at risk for bronchopulmonary dysplasia. These factors might favor deposition of fibrin in the lungs, thus promoting respiratory failure.
In the present study, the plasma kinin-kallikrein system is more activated in the severe RDS than in the mild/moderate RDS group as indicated by higher kallikrein activity and lower PKKI values in the severe group. Activation of the plasma kinin-kallikrein system has been previously shown by Saugstad et al.(9, 10) in preterm infants with severe RDS. Formation of kallikrein results in production of bradykinin(15) that produces vasodilatation by endothelial release of nitric oxide and prostaglandin I2(15) and causes extravasation of macromolecules(39). Bradykinin-induced pulmonary vasodilation is well documented in the fetal lamb(40), whereas concentrations of the main bradykinin-inhibiting enzyme in the lung, angiotensin-converting enzyme, is low in newborn lambs(41). We, therefore, suggest that bradykinin promotes the development of protein-rich edema in preterm infants with RDS thus increasing disease severity.
Products of clotting (thrombin, fibrin) and kinin-kallikrein (bradykinin) activation might have contributed to decreased neutrophil counts in the severe RDS group. In thrombin-mediated lung injury, thrombin induces neutrophil chemotaxis, endothelial adherence, and entrapment in fibrin microthrombi in the pulmonary microcirculation(13). Bradykinin causes migration of neutrophils to sites of injury(15). In severe RDS, neutrophils accumulate in the lungs and release products that are involved in destruction of lung connective tissue and breakdown of pulmonary vascular endothelium(42–44).
The decreased platelet count in the severe RDS infants agrees with previous findings in RDS(6, 45). In ARDS patients, lower platelet counts indicate platelet entrapment in the lungs(46) that is probably mediated by thrombin and platelet-neutrophil interactions(47). Once being entrapped in the lungs platelets will aggregate and release bioactive substances such as thromboxane that are able to contribute to pulmonary dysfunction(46). Although increased thromboxane levels have been observed in tracheal effluent of preterm infants with RDS(48), the role of platelets in the pathogenesis of RDS remains speculative.
In this study we did not find differences between both RDS groups for prenatal factors that could influence the activation of clotting, fibrinolysis, kinin-kallikrein, and the neutrophil and platelet counts (see Table 1). Furthermore, within each RDS group we did not observe influence of these prenatal factors and several postnatal factors(hyperbilirubinemia, phototherapy, blood transfusions, and umbilical catheters) on the parameters we studied. However, one should be aware of the rather small numbers of infants that have been compared. Birth weight and gestational age were not correlated with the parameters that were studied. However, higher T-AT III complex concentrations and lower platelet counts at 6-12 h from birth were correlated with lower 1- and 5-min Apgar scores and lower arterial umbilical pH values, whereas higher t-PA concentrations at 6-12 h from birth were correlated only with lower Apgar scores. Lower Apgar scores and lower arterial umbilical pH values indicate perinatal hypoxemia and acidosis that are known to stimulate endothelial cells to release tissue factors and t-PA, thus activating clotting and fibrinolysis, respectively(49, 50). Furthermore, higher Fio2 and PIP values were correlated with more activation of clotting, fibrinolysis, and kinin-kallikrein throughout the study period. Hyperoxia and positive pressure ventilation cause lung tissue destruction, which can be accompanied by factor XII and kallikrein activation(15, 51), as has been observed in severe neonatal RDS(6, 9). Factor XII activity contributes to clotting and fibrinolytic activity(15, 51). High Fio2 and PIP values may promote activation of plasma proteins, but may, in turn, indicate increased ventilatory requirements representing increase RDS severity due to this activation process.
In this study, we have demonstrated that during the first 5 d of life clotting, fibrinolysis, and kinin-kallikrein become simultaneously activated in preterm infants with severe RDS. This activation process is accompanied by a decrease of circulating neutrophils and platelets. We suggest that hypoxemia and acidosis due to perinatal asphyxia and pulmonary tissue injury due to artificial ventilation promote this activation process. Based on the correlations that we found we hypothesize that the degree of activation of clotting, fibrinolysis, and kinin-kallikrein contributes to the degree of RDS severity. Future studies should be directed to elucidate the nature of this activation process in relation to the development of inflammation and proteinaceous edema in the lungs of preterm infants with RDS.
Abbreviations
- RDS:
-
respiratory distress syndrome
- ARDS:
-
acute respiratory distress syndrome
- T-AT III:
-
thrombin-antithrombin III
- t-PA:
-
tissue-type plasminogen activator
- PKKI:
-
plasma kallikrein inhibitory activity
- Fio2:
-
fraction of inspired oxygen
- PIP:
-
peak inspiratory pressure
- VEI:
-
ventilator efficiency index
- a/APo2:
-
arterial/alveolar oxygen tension ratio
- HELLP:
-
hemolysis elevated liver enzymes, and low platelet count
- CPAP:
-
continuous positive airway pressure
- CPP:
-
cryoglobulin poor plasma
References
Jefferies AL, Coates G, O'Brodovich H 1984 Pulmonary epithelial permeability in hyaline membrane disease. N Eng J Med 311: 1075–1080.
O'Brodovich HM, Coates G 1988 Pulmonary edema in respiratory distress syndrome and bronchopulmonary dysplasia. In: Merritt TA, Northway WH, Boynton BR (eds) Bronchopulmonary Dysplasia, Blackwell Scientific Publications, Boston, pp 143–159.
Jobe A, Jacobs H, Ikegami M, Berry D 1985 Lung protein leaks in ventilated lambs: effect of gestational age. J Appl Physiol 58: 1246–1251.
Nilsson R, Grossmann G, Robertson B 1978 Lung surfactant and the pathogenesis of neonatal bronchiolar lesions induced by artificial ventilation. Pediatr Res 12: 249–255.
Berry D, Jobe A, Ikegami M 1991 Leakage of macromolecules in ventilated and unventilated segments of preterm lamb lungs. J Appl Physiol 70: 423–429.
Brus F, van Oeveren W, Okken A, Bambang Oetomo S 1994 Activation of the plasma clotting, fibrinolytic and kinin-kallikrein system in preterm infants with severe idiopathic respiratory distress syndrome. Pediatr Res 36: 647–653.
Peters M, Ten Cate JW, Breederveld C, De Leeuw R, Emeis J, Koppe J 1984 Low antithrombin III levels in neonates with idiopathic respiratory distress syndrome: poor prognosis. Pediatr Res 18: 273–276.
Schmidt B, Vegh P, Weitz J, Johnston M, Caco C, Roberts R 1992 Thrombin/antithrombin III complex formation in the neonatal respiratory distress syndrome. Am Rev Respir Dis 145: 767–770.
Saugstad OD, Buo L, Johansen HT, Roise O, Aasen AO 1992 Activation of the plasma kallikrein-kinin system in respiratory distress syndrome. Pediatr Res 32: 431–435.
Saugstad OD, Harvie A, Langslet A 1982 Activation of the kallikrein-kinin system in premature infants with respiratory distress syndrome (RDS). Acta Paediatr Scand 71: 965–968.
Stark CR, Abramson D, Erkan V 1968 Intravascular coagulation and hyaline membrane disease of the newborn. Lancet 2: 1180–1181.
Corrigan JJ, Jeter MA 1992 Tissue-type plasminogen activator, plasminogen activator inhibitor, and histidine-rich glycoproteins in stressed human newborns. Pediatrics 89: 43–46.
Malik AB, Horgan MJ 1987 Mechanisms of thrombin-induced lung vascular injury and edema. Am Rev Respir Dis 136: 467–470.
Haynes JB, Hyers TM, Giclas PC, Franks JJ, Petty TL 1988 Elevated fibrin degradation products in ARDS. Am Rev Respir Dis 122: 841–847.
Stewart JM 1993 The kinin system in inflammation. Agents Actions Suppl 42: 145–157.
Giedion A, Haefliger H, Dangel P 1973 Acute pulmonary x-ray changes in hyaline membrane disease treated with artificial ventilation and positive end expiratory pressure. Pediatr Radiol 1: 145–152.
Koenig JM, Christensen RD 1991 Incidence, neutrophil kinetics, and natural history of neonatal neutropenia associated with maternal hypertension. N Eng J Med 321: 557–562.
Brazy JE, Grimm JK, Little VA 1982 Neonatal manifestations of severe maternal hypertension occurring before the thirty-sixth week of pregnancy. J Pediatr 100: 265–271.
Jansen NJG, van Oeveren W, van Vliet M, Stoutenbeek CP, Eysman L, Wildevuur Ch RH 1991 The role of different types of corticosteroids in the inflammatory mediators in cardiopulmonary bypass. Eur J Cardiothorac Surg 5: 211–217.
Levene MI, Williams JL, Fawer CL 1985 Ultrasound of the infant brain. Clin Dev Med 92: 000–000.
Gu YJ, Obster R, de Haan J, Gallandat Huet RCG, van Oeveren W 1992 Biocompatibility of leukocyte removal filters during bedside leukocyte filtration of red cell concentrates. Transfus Sci 13: 467–472.
van Lingen RA, Hofhuis WDJ, Dekker I, Baerts W, Hählen K, Sauer PJJ 1992 The effect of heparin in arterial catheters on the coagulation in preterm infants. J Perinat Med 20: 39–46.
Notter RH, Egan EA, Kwong MS, Holm BA, Shapiro DL 1985 Lung surfactant replacement in premature lambs with extracted lipid from bovine lung lavage: effects of dose, dispersion technique and gestational age. Pediatr Res 19: 569–577.
Redmond CR, Loe WA, Bartlett RH, Arensman RM 1988 Extracorporeal membrane oxygenation. In: Goldsmith JP, Karotkin EH, Barker S(eds) Assisted Ventilation of the Neonate. WB Saunders, Philadelphia, pp 200–212.
van der Kamp KWHJ, van Oeveren W 1994 Beta-factor XII a and kallikrein generation in plasma during incubation with biomaterials. J Biomed Mater Res 28: 349–352.
Saugstad OD, Aasen AO, Guldvog I, Lium B, Lyngaas K, Amundsen E 1982 Changes of components of the plasma kallikrein-kinin system during experimental lung insufficiency in dogs. Acta Chir Scand Suppl 509P: 61–67.
Guldvog I, Saugstad OD, Aasen AO, Dale J, Lium B, Larsbraaten M, Lyngaas K, Amundsen E 1980 Blood cells and coagulation during experimental lung insufficiency in dogs. Acta Chir Scand Suppl 499P: 131–139.
Aasen AO, Saugstad OD, Lium B, Guldvog I, Lyngaas K, Larsbraaten M, Amundsen E 1980 Plasma antiplasmin activities in experimental lung insufficiency. Acta Chir Scand Suppl 499P: 113–121.
Sarnaik AP, Lieh-Lai M 1994 Adult respiratory distress syndrome in children. Pediatr Clin North Am 41: 337–364.
Palta M, Gabbert D, Fryback D, Widjaja I, Peters ME, Farrell P, Johnson J 1990 Development and validation of an index for scoring baseline respiratory disease in the very low birth weight neonate. Pediatrics 86: 714–721.
Cooper JA, Feustel PJ, Line BR, Malik AB 1986 Pulmonary epithelial clearance of 99mTc-DTPA after thrombin-induced pulmonary microembolism. Am Rev Respir Dis 134: 734–738.
Busing CM, Bleyl U 1974 Oxygen induced pulmonary hyaline membranes (PHM) and disseminated intravascular coagulation (DIC). Virchows Arch 363: 113–122.
Fuchimukai T, Fujiwara T, Takahashi A, Enhorning G 1987 Artificial pulmonary surfactant inhibited by proteins. J Appl Physiol 62: 429–437.
Ueda T, Ikegami M, Jobe A 1994 Surfactant subtypes. In vitro conversion, in vivo function, and effects of serum proteins. Am J Respir Crit Care Med 149: 1254–1259.
Corrigan JJ 1992 Normal hemostasis in the fetus and newborn coagulation: In: Polin RA, Fox WW (eds) Fetal and Neonatal Physiology, Vol 2. WB Saunders, Philadelphia, pp 1368–1371.
Liu J, Gurewich V 1993 Substrate inhibition of fibrin-dependent plasminogen activation by tissue-type plasminogen activatory. J Biol Chem 268: 12257–12259.
Viscardi RM, Broderick K, Sun CJ, Yale-Loehr AJ, Hessamfar A, Taciak V, Burke KC, Koenig KB, Idell S 1992 Disordered pathways of fibrin turnover in lung lavage of premature infants with respiratory distress syndrome. Am Rev Respir Dis 146: 492–499.
Idell S, Kumar A, Koenig KB, Coalson JJ 1994 Pathways of fibrin turnover in lavage of premature baboons with hyperoxic lung injury. Am J Repir Crit Care Med 149: 767–775.
Gao XP, Mayan WG, Conlon JM, Rennard SI, Rubinstein I 1993 Mechanisms of T-kinin-induced increases in macromolecule extravasation in vivo. J Appl Physiol 74: 2896–2903.
Frantz E, Soifer SJ, Clyman RI, Heyman MA 1989 Bradykinin produces pulmonary vasodilatation in fetal lambs: role of prostaglandin production. J Appl Physiol 67: 1512–1517.
Pitt BR, Lister G 1984 Kinetics of pulmonary angiotensin-converting enzyme activity in concious developing lambs. J Appl Physiol 57: 1158–1166.
Merritt TA, Cochrane CG, Holcomb K, Bohl B, Hallman M, Strayer D, Edwards DK 1983 Elastase and α-1-proteinase inhibitor activity in tracheal aspirates during respiratory distress syndrome. J Clin Invest 72: 656–666.
Speer CP, Ruess D, Harms K, Herting E, Gefeller O 1993 Neutrophil elastase and acute pulmonary damage in neonates with severe respiratory distress syndrome. Pediatrics 91: 794–799.
Groneck P, Götze-Speer B, Oppermann M, Eiffert H, Speer CP 1994 Association of pulmonary inflammation and increased microvascular permeability during the development of bronchopulmonary dysplasia: a sequential analysis of inflammatory mediators in respiratory fluids of high-risk preterm neonates. Pediatrics 93: 712–718.
Kohelet D, Perlman M, Hanna G, Ballin A 1990 Reduced platelet counts in neonatal respiratory distress syndrome. Biol Neonate 57: 334–342.
Heffner JE, Sahn SA, Repine JE 1987 The role of platelets in the adult respiratory distress syndrome. Culprits or bystanders? Am Rev Respir Dis 135; 482–492
Renesto P, Chignard M 1993 Enhancement of cathepsin G-induced platelet activation by leukocyte elastase: consequence for neutrophil-mediated platelet activation. Blood 82: 139–144.
Le Guennec JC, Lauziere M, Black R, Sirois P 1991 Effects of indomethacin on prostaglandin E2 and thromboxane B2 contents of tracheal lavage fluids in premature infants. Inflammation 15: 55–59.
Nemerson Y 1992 The tissue factor pathway of blood coagulation. Semin Hematol 29: 170–176.
Collen D 1980 On the regulation and control of fibrinolysis. Thromb Haemostas 43: 77–89.
Kaplan AP, Silverberg M 1987 The coagulation-kinin pathway of human plasma. Blood 70: 1–15.
Acknowledgements
The authors thank Johan Haan for his excellent technical assistance and Miep Helfrich for correction of the manuscript.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brus, F., Van Oeveren, W., Okken, A. et al. Disease Severity Is Correlated with Plasma Clotting and Fibrinolytic and Kinin-Kallikrein Activity in Neonatal Respiratory Distress Syndrome. Pediatr Res 41, 120–127 (1997). https://doi.org/10.1203/00006450-199701000-00019
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199701000-00019
This article is cited by
-
Activatie van plasma-eiwitten en bloedcellen bij het neonataal respiratoir distress-syndroom: pathogenetische en therapeutische aspecten
Tijdschrift voor kindergeneeskunde (2000)