Abstract
Surfactant instillation may affect systemic and pulmonary hemodynamics. The aim of this study was to investigate whether this effect is specific to surfactant or if it can be triggered by instillation of the same volume of saline. Piglets 3-5-d-old were subjected to repeated lung lavage using 20 mL/kg 0.9% saline until the partial pressure of arterial O2 was <10 kPa and partial pressure of arterial CO2 was between 4.0 and 6.0 kPa with fraction of inspired oxygen (Fio2) 1.0 and peak inspiratory pressure 25 cm H2O. Porcine surfactant 200 mg/kg (80 mg/mL) or the same volume of 0.9% saline was instilled into the lungs through a feeding catheter entered through the endotracheal tube. Mean arterial blood pressure, pulmonary artery pressure, and cardiac output were measured continuously. There was a significant decrease in mean arterial blood pressure from 67 (±13) mm Hg to 52 (±18) mm Hg (p < 0.05) 210 s after instillation of surfactant. Systemic vascular resistance decreased from 0.42 (±0.18) to 0.34 (±0.18) mm Hg × mL-1 × min × kg(p < 0.05) from 0 min to 180 s after instillation of surfactant. In the group receiving saline instillations there were no significant changes in mean arterial blood pressure or systemic vascular resistance. A transient but significant increase in mean pulmonary artery pressure was seen 120 s after instillation in both groups with a return to presurfactant level 240 s after instillation. Pulmonary vascular resistance increased transiently and significantly only in the group receiving surfactant. We conclude that porcine surfactant causes a decrease in systemic vascular resistance, resulting in a decrease in mean arterial blood pressure in newborn lung-lavaged piglets not seen after instillation of the same volume of saline.
Similar content being viewed by others
Main
During the last 5 y surfactant replacement therapy has significantly improved outcome after premature birth(1, 2). Few severe side effects have been reported, but acute systemic hemodynamic effects occurring after surfactant replacement have been observed both in clinical(3–7) and experimental(8, 9) studies. We have recently demonstrated that MABP decreases after surfactant instillation in newborn piglets, independent of preceding hypovolemia or hypoxemia(10), and that this decrease is caused by a decrease in SVR.
A small but significant increase in incidence of pulmonary hemorrhage after treatment with exogenous surfactant has been found(11) often attributed to an increase in pulmonary blood flow. PAP and pulmonary hemodynamics in children with RDS have been investigated using ultrasound-Doppler technique(12–14). These studies have shown that PAP in early RDS is not significantly higher than in controls without RDS, but that in severe RDS PAP fails to decrease after birth as it does in healthy newborn infants. The effect of surfactant on pulmonary hemodynamics has also been evaluated in clinical(15–18) and experimental(19–21) studies, but the results have been conflicting and partially disappointing.
The purpose of the present study was to perform a detailed investigation of the acute effects of porcine surfactant instillation on systemic and pulmonary circulation using a computer-based system for continuous registration of hemodynamic variables. We hypothesized that the hemodynamic effects seen after porcine surfactant instillation are not caused by the procedures or instillation of a fluid bolus dose. These effects would therefore be observed only after instillation of porcine surfactant, and not after instillation of the same volume of saline.
METHODS
Surgical preparation. Twenty-one 3-5-d-old piglets were obtained from a local farmer on the day of the experiments. Anesthesia was achieved with pentobarbital sodium, 20 mg/kg intraperitoneally, and pethidine, 2.5 mg/kg intramuscularly, followed by pentobarbital sodium, 20 mg/kg i.v, and pethidine, 2.5 mg/kg i.v. Lidocaine 1% was used for incisions. A continuous pentobarbital infusion (6 mg/kg/h) was administered in the left jugular vein throughout the experiment. Additional pentobarbital was given if necessary during surgery. During the experiment a peripheral i.v. infusion containing 0.7% NaCl and 1.25% glucose was given at a rate of 10 mL/kg/h. Heart rate was monitored continuously via skin electrodes, and rectal temperature was kept between 38.0 and 39.5 °C with a heating blanket and a radiant heating lamp.
A tracheostomy was established, and a 3.5-mm cuffed endotracheal tube was inserted. The tube was connected to a Bourns BP 200 pressure regulated infant ventilator. The piglets were mechanically ventilated at 30-40 breaths/min. PIP was adjusted to maintain Paco2 between 4.0 and 6.0 kPa. During surgery Fio2 was set between 0.21 and 0.30 to maintain Pao2 between 8.0 and 12.5 kPa.
Polyethylene catheters (Portex PE-50; ID 0.58 mm) were placed in the left femoral artery and in the left common carotid artery and jugular vein. All catheters were flushed regularly with heparinized saline (4 U/mL). The carotid artery catheter was connected to a transducer, and blood pressure was recorded continuously with a pressure transducer (model AE840 SensoNor, Horten, Norway) connected to a signal amplifier (Gould Transducer or Universal, Cleveland, OH). The femoral artery catheter was used for arterial blood samples. A catheter was also inserted into the left femoral vein and advanced to the proximal part of the inferior caval vein for measurement of CVP in four animals (two in each group).
A thoracotomy was performed, the pericardium was opened, and the facies around the common pulmonary artery were removed. An 8-mm ultrasonic transit time flowprobe (Cardio Med, Medi-Stim, Oslo, Norway) was fitted around the common pulmonary artery for measurements of CO. The flowprobe diameter was chosen not to restrict the diameter of the pulmonary artery, and the positioning was checked by direct visual inspection before every experiment.
A catheter (Vygon, outside diameter 1.2 mm, Écouen, France) was then introduced through the anterior wall of the right ventricle of the heart and advanced through the pulmonary valve into the common pulmonary artery. Correct placement of the catheter was verified by blood pressure tracing pattern. The catheter was used for measurement of PAP.
In three pilot animals and three animals included in the study a small incision was made in the wall of the left atrium to insert the tip of a 5 F Schwann-Ganz catheter. A ligature was placed around the catheter to avoid bleeding. The Schwann-Ganz catheter was then connected to a pressure transducer, and pressure in the left atrium was measured continuously. Because of technical difficulties (complications due to bleeding and difficulties with keeping the catheter open), this procedure was not performed throughout the study.
Surfactant deficiency was induced by lung lavage performed through the endotracheal tube with 20 mL/kg, 0.9% saline heated to 38 °C with the piglet in the supine position. PEEP was set to 2 cm H2O. PIP was gradually increased to 25 cm H2O. I:E ratio was 1:2 and Fio2 after lavage was 1.0. Lavages were repeated until Pao2 was <10.0 kPa with Paco2 > 4.0 kPa. In the surfactant group lavage was performed 12 (±3) times and in the control group 16 (±6) times (NS). Arterial blood gases were measured 10 min after the end of lavage. Occasionally there was some improvement in blood gases after the end of lavage, and ventilator adjustments were made to avoid Pao2 outside the predefined range between 8.0 and 12.5 kPa.
Two animals in the surfactant group and three in the saline group were given 10 mL/kg 4% albumin to correct a MABP after lavage less than 55 mm Hg. In the other animals albumin was not necessary due to a MABP > 55 mm Hg.
Fifteen minutes before instillation the animals were randomized to receive either porcine surfactant (200 mg/kg, 80 mg/mL) or 0.9% saline (2.5 mL/kg). Four animals were excluded before randomization due to lethal hemorrhage, pneumothorax, peri/myocarditis (judged macroscopically from pericardial thickening, purulent pericardial effusion, and necrotic lesions in the myocardium), and blood pressure <50 mm Hg despite albumin infusion. One animal in the surfactant group was excluded after the experiment due to computer logging failure. Thus the experiment contains two groups with eight animals in each. Measurements of PAP were only successful in seven animals in each group due to plugging of catheters.
Sixty minutes after the end of lavage, porcine surfactant or saline was instilled in two bolus doses through a feeding catheter inserted into the lumen of the endotracheal tube. Time of disconnection from the ventilator to perform each instillation did not exceed 10 s. After the first dose the piglet was manually ventilated for three to five respiratory cycles and then reconnected to the ventilator at the same Fio2 and PIP as before instillation. Sixty seconds after the first dose the procedure was repeated for the second dose. PIP or Fio2 was not increased after instillation and if Pao2 started to increase Fio2 was first reduced, and then Fio2 and PIP were reduced in response to increasing Pao2 and decreasing Paco2. No adjustments were made to I:E-ratio, frequency, or PEEP.
Blood samples. Blood samples from the left femoral artery were taken regularly during surgery, 5 min before instillation, and at 150 s, and 5, 10, 15, and 20 min after instillation. Temperature-corrected blood gases were measured with an AVL 945 automatic blood gas system (Schaffhausen, Switzerland).
CI was calculated as CO/weight. CVPs measured in four piglets during and after instillation were stable at a mean value of 2 mm Hg despite changes in MABP. SVR was therefore calculated without including CVP using the formula MABP/CI. Pressure in the left atrium was successfully measured in three pilot animals and in two animals included in the study. Of these, four received surfactant and one received saline. Mean pressure was 5 mm Hg, varying less than 1 mm Hg during and after instillation. PVR was therefore calculated as PAP/CI. Not subtracting LAP from PAP causes a small overestimation of absolute PVR, but has a minimal effect when relative changes are studied. The stability of CVP and LAP during instillation was later confirmed in seven animals receiving surfactant with mean values for CVP and LAP of 2 and 3 mm Hg, respectively (our unpublished results).
Data collection and analysis. All blood pressure, flow, and heart rate data were logged into a computer using a software system developed for logging of data (Work Bench PC for Windows, Sunnyvale, CA) and using a logging frequency of 1 Hz. CI, SVR, and PVR were continuously calculated in the computer system during the experiment. The exact time point for start of surfactant instillation was marked in the data file, so that comparisons between piglets and groups could be accurately performed. Before statistical evaluation, mean values for the logged and calculated variables were calculated in blocks of 30 s (sum of 30 samples/30) in each animal. The mean values in each group were calculated by summing the block means for each animal/number of animals. The last block before disconnecting the piglet from the ventilator served as reference for statistical analysis of effects of instillation, in figures called 0 min, and the first block evaluating the effect of instillation is calculated for the next 30 s. This means that the block value called 30 s reflects the mean of 1-30 s and 20 min reflects the mean of values between 19 min, 31 s, and 20 min.
Effects of lung-lavage was evaluated by comparing prelavage with 0 min. The stability of the model was evaluated by comparing -5 min with 0 min. Previous studies revealed that the maximum effect on MABP was seen 2-5 min after surfactant instillation(10). We therefore considered the time point of maximum change within the first 5 min as judged from the mean curve in each group to be an appropriate estimate of the hemodynamic effects of instillation. To evaluate possible prolonged effects of instillation, 20 min after instillation was selected. Differences between groups were evaluated by comparing Δ values within each group for the effect. Wilcoxon signed rank test was used for evaluation of the effect of lung lavage and for single paired comparisons of ventilator settings and blood gases. Friedman's test for nonparametric repeated measurements was used within each group to study the effects of instillation starting at -5 min. Dunnet's test was used as post hoc test when a significant effect was detected by the Friedman test. The Mann-Whitney U test was used for unpaired comparisons between groups. All data are given as mean ± SD. A p value<0.05 was considered statistically significant.
Approval. The experimental protocol was approved by the hospital's ethics committee for animal studies.
RESULTS
Ventilator settings are shown in Table 1. Both PIP and Fio2 could be significantly reduced in the surfactant group within 20 min after surfactant instillation (p < 0.05), whereas no changes occurred in the saline group. PIP and Fio2 were significantly lower in the surfactant group than in the saline group at 20 min (p < 0.05).
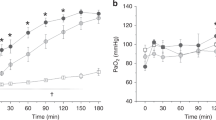
Pao2 (Fig. 1) did not change significantly in any group after instillation. Paco2 increased significantly from 4.6(±0.6) kPa to 5.3 (±0.7) kPa in the surfactant group at 150 s(p < 0.05), but returned to presurfactant levels at 5 min. In the saline group Paco2 was 4.8 (±1.0) kPa before instillation and did not change significantly after instillation.
MABP (Fig. 2) was not significantly different in the two groups before instillation, and no significant changes were seen in any of the groups from -5 to 0 min. MABP decreased from 67 (±13) mm Hg to 52(±18) mm Hg in the surfactant group 210 s after instillation(p < 0.05). In the saline group MABP was 68 (±10) mm Hg at 0 min and 61 (±19) mm Hg 150 s after instillation (NS) (ΔMABP between groups NS). Twenty minutes after instillation MABP was not significantly different from 0 min in the surfactant group, but a significant decrease was seen in the saline group.
CI (Fig. 3) did not change significantly in any group from prelavage to 0 min or from -5 min to 0 min. CI at 0 min in the surfactant group was 170 (±51) mL × min-1 × kg-1 and in the saline group 208 (±69) mL × min-1 × kg-1(p = 0.40). One hundred and twenty seconds after instillation, CI in the surfactant group was 151 (±39) mL × min-1 × kg-1 (NS) and in the saline group 193 (±54) mL × min-1 × kg-1 (NS), ΔCI between the groups did not change significantly.
SVR (Fig. 4) increased significantly from prelavage to 0 min in the surfactant group only. No significant changes were seen from -5 min until 0 min. No significant differences were detected between the two groups at 0 min (p = 0.5). In the surfactant group SVR decreased from 0.42 (±0.18) mm Hg × mL-1 × min × kg at 0 min to 0.34 (±0.18) mm Hg × mL-1 × min × kg(p < 0.05) at 180 s. In the saline group SVR at 0 min was 0.37(±0.16) mm Hg × mL-1 × min × kg and at 210 s 0.35 (±0.16) mm Hg × mL-1 × min × kg (NS), The p value for comparison of ΔSVR in the two groups was 0.06. At 20 min SVR was not significantly different from SVR at 0 min in any group.
Systemic vascular resistance. Closed circles = surfactant group (n = 8), open circles = saline group (n = 8). † = p < 0.05 vs prelavage. * =p < 0.05 vs 0 min. SVR increased significantly after lavage in the surfactant group only and decreased significantly 180 s after instillation. No changes occurred in the saline group.
Heart rate was stable in both groups during the first 5 min after instillation starting at 215 (±52) beats/min in the saline group and 220 (±47) beats/min in the surfactant group, and then varying between 211 (±51) and 217(±43) beats/min and 211(±47) and 225(±48) beats/min in the two groups, respectively.
PAP (Fig. 5) increased significantly in the surfactant group only from prelavage to 0 min (p < 0.05). From -5 to 0 min, no significant changes occurred in any of the groups, and PAP at 0 min was 25(±4) mm Hg in both groups. At 120 s after the start of instillation, PAP had increased to 32 (±7) mm Hg (p < 0.05) in the surfactant group. In the saline group maximum PAP 120 s after instillation was 26 (±5) mm Hg (p < 0.05). Comparing ΔPAP between groups showed no significant difference in response (p = 0.09). At 5 min PAP had returned to the preinstillation level and remained there.
PVR (Fig. 6) increased significantly from prelavage to 0 min in both groups (p < 0.05). No significant differences were seen between -5 and 0 min. PVR in the surfactant group before instillation was 0.15 (±0.05) mm Hg × mL-1 × min × kg and in the saline group 0.14 (±0.07) mm Hg × mL-1 × min× kg (NS). It increased significantly to 0.19 (±0.04) mm Hg× mL-1 × min × kg (p < 0.01) 120 s after instillation in the surfactant group and returned to preinstillation levels at 5 min. No significant change in PVR was seen in the saline group. ΔPVR was significantly higher in the surfactant group (p < 0.01). At 20 min PVR was not significantly different from PVR at 0 min in any group.
DISCUSSION
This study demonstrates that the decrease in MABP and SVR after surfactant instillation previously demonstrated in newborn piglets(10) cannot be explained solely by the procedures or instillation of fluid into the lungs. On the other hand, surfactant seems to prevent a decrease in MABP seen in the saline group 20 min after instillation. In the pulmonary circulation there is a brief increase in PVR in the surfactant group only.
The model used in this study is in many respects different from the situation seen in premature infants. The lung lavage model does not reproduce all aspects of RDS, but mimics the most important characteristics with an impaired lung function due to surfactant deficiency. The response to surfactant instillation in the model is somewhat slower than the response described in both clinical studies and other experimental studies, with the improvement in oxygenation starting about 5 min after instillation. This may be an effect of the thoracotomy causing more extensive atelectasis and a less rapid expansion of the lungs. However, 20 min after instillation a significant reduction in PIP and Fio2 is present only in the group receiving surfactant.
Models with an open chest and puncture of the myocardium and the atria have been used to study the effects of oxygen free radicals on pulmonary circulation(22) and to study effects of changes in afterload on right ventricular function in newborn piglets(23). Compared with our previous work(10) that was carried out using a closed chest preparation, the decreases in MABP and SVR after surfactant instillation were less pronounced. MABP before instillation was similar in the two studies, but CO was considerably lower in the present study. Because MABP was similar in the two studies, this indicates a higher SVR before instillation in the present experiment. This may have influenced the magnitude of the vasodilatation and the decrease in MABP.
Despite important differences between the clinical situation and the experimental model, they both have in common the effect of porcine surfactant instillation on MABP. This effect is the main subject of this study, and the mechanism causing it is not understood in any of these situations. We cannot prove that the mechanism causing the effect is the same in both situations. However, it seems unlikely that the same pharmacologic agent should cause the same effect via different mechanisms when changes in CO are not involved. Because no decrease in SVR was seen in the saline group, the effect cannot be explained by the extensive surgical interventions alone.
With respect to pulmonary circulation, 3-d-old piglets have almost completed the initial transition from fetal pulmonary circulation(24). The pretreatment pulmonary artery pressures in our model are therefore considerably lower than the pulmonary pressures observed in neonates immediately after delivery(14). Still PVR in both groups increased after lavage, resembling some of the vasoconstriction seen in persistent pulmonary hypertension in RDS.
We have assumed that measurements of flow in the common pulmonary artery equals left ventricular output. If a shunting across foramen ovale occurred, flow on the right side would not equal left ventricular output. However, we consider a left to right shunt through the foramen ovale unlikely, since this had to be attributed to either anatomical malformations or a very high left atrial pressure sufficient to dilate the atrium. As we have demonstrated, left atrial pressure was low in our preparation and remained unchanged despite surfactant instillation. A right to left shunt is therefore more likely to occur due to an increase in PAP, as seen immediately after instillation. In a study of 3-5-d-old piglets(23) PAP was increased stepwise from 23 mm Hg. A significant right to left shunt did not occur until mean PAP was 52 mm Hg, 20 mm Hg higher than the maximum PAP observed in this study. Therefore shunting across the foramen ovale is very unlikely in our preparation. Measuring left ventricular output(10) resulted in the same conclusion as reported here with no change in CO seen after instillation. Ductus arteriosus in 3-5-d-old piglets is functionally closed(10, 23, 24).
The possible effects of pentobarbital anesthesia on hemodynamics has previously been discussed(10). The accurate registrations with time allow us to investigate in detail the course of the changes taking place. The effect on PAP and PVR started immediately after instillation of the first bolus of surfactant and reached a maximum after 120 s. Later it returned to the preinstillation level within 5 min. The effect on the systemic circulation was delayed compared with pulmonary circulation. During the first 90 s (the period of disconnection and instillation) there were no or only minor changes in MABP and CI in both groups. At 120 s CI was 19 mL/kg (surfactant) and 15 mL/kg (saline) lower than at 0 min. CI in both groups and MABP in the saline group then stabilized. From 90 s until 210 s MABP decreased only in the group receiving surfactant. As seen in Fig. 4 this decrease in MABP is explained by a systemic vasodilatation not seen in the saline group.
Recently Lundstrøm et al.(25) reported measurements of left ventricular output in infants by Doppler technique simultaneously with Curosurf (Chiesi Pharmaceuticals Inc.) instillation and found that, despite an increase in left ventricular output of 30%, MABP decreased. Although the status of ductus arteriosus was not evaluated, the increase in left ventricular output probably reflects a compensatory mechanism due to a decrease in SVR.
A limited number of studies on the effect of surfactant on pulmonary circulation have been published, and no studies have reported in detail the acute effects within the first 5 min as in our study. Jobe et al.(20) studied systemic and pulmonary hemodynamics in premature lambs. There was a decrease in PAP in those animals with severe RDS the first 20 min after instillation, but 1 h after instillation it was no longer different from baseline. In their model, using sheep surfactant, MABP decreased about 10 mm Hg immediately after instillation, but this decrease failed to reach significance. Clyman et al.(19) found no differences in systemic or pulmonary hemodynamic variables in premature lambs receiving surfactant compared with nontreated controls, but an increase in the PVR/SVR ratio with time occurring in nontreated animals was avoided in animals receiving surfactant. Vidyasagar et al.(21) found that in immature baboons pulmonary blood flow increased 3 h after instillation of Surfactant TA and returned to baseline level at 7 h.
Two studies in infants on the effect of Exosurf (Burroughs Wellcome) on pulmonary circulation have been published(16, 17). They both found that Exosurf decreased PAP after surfactant instillation, whereas Halliday et al.(15) and Bloom et al.(18) did not find any significant influence of Curosurf on pulmonary hemodynamics.
The cause of the increase in PVR 120 s after the start of instillation only in the group receiving surfactant is not obvious. It may be due to a transient decrease in Pao2 in connection with the instillation, but Pao2 measured 150 s after instillation did not indicate a different response in the two groups in oxygenation. The minor increase in Paco2 seen at 150 s may have contributed to the increase in PVR, but the increase is small and could probably not explain the increase in PVR. A possible effect of surfactant directly on the pulmonary endothelium cannot be excluded, but it requires a very rapid transition from the alveoli to the pulmonary circulation. The increase in the surfactant group may also be related to different effects on pulmonary mechanics in the two groups.
The effect seen on SVR in this study is caused by a specific effect of porcine surfactant in the lungs rather than the stress related to the procedures or instillation of a fluid bolus dose. In infants MABP decrease has not been observed after treatment with Exosurf(26, 27). Survanta (Abbott Laboratories) has been studied in one trial without detection of blood pressure effects, but brief effects may have gone unnoticed, because MABP was averaged over 5 min(27). One report on Alveofact (Boehringer Mannheim)(28) measured MABP 10 min after instillation and found no changes, but this design would not detect rapid changes within the first 5 min. Due to the limited number of studies of other natural surfactants, it is not possible to conclude whether the effects observed is specific for Curosurf, or if it is a general feature of natural surfactants.
We did not observe a pronounced hypoxemia immediately after instillations, but due to noncontinuous measurements this possibility cannot be excluded. Our experience from other experiments with induction of severe hypoxemia(29) is that the almost uniform reaction in newborn piglets to a sudden onset of hypoxemia is an increase in MABP for a few minutes and thereafter a slow decrease over a period of 30-60 min. Although these two models are not directly comparable, this indicates that a brief and moderate hypoxemia does not produce the hemodynamic effects seen in our model.
A decrease in systemic vascular resistance in dogs perfused with a constant flow has been reported after a moderate inflation of the lungs(30–32). This effect was mediated via sympathetic vagal afferents from the lungs in response to inflation, causing an inhibition of sympathetic vagal activity in efferent fibers to peripheral arterioles and resulting in a decrease in vascular tone. Pulmonary inflation also resulted in an increase in vascular capacitance in the venous part of the splanchnic circulation(32). The only way to inflate the lungs before the introduction of surfactant therapy has been to increase PIP or PEEP. This causes a decrease in CO, and then reflexively an increase in SVR to maintain a stable MABP(33). This increase in SVR would then override and mask the inflation reflex. Therefore a clinical significance of this inflation reflex has previously not been noted. The introduction of surfactant therapy may have introduced a mechanism for inflating the lungs without major effects on CO. Both experimental(34) and clinical studies(35–37) have clearly demonstrated that the immediate effect of instillation of natural surfactant is an increase in FRC and also improvement in static compliance(38–40). Recently Sindelar et al.(41) found that surfactant instillation in surfactant-depleted cats increased activity in slowly adapting pulmonary stretch receptors, which are the receptors responsible for eliciting the lung inflation reflex.
Based on the rapid improvements in FRC and compliance reported by others, and the systemic vasodilatation reported by us, we speculate that the main reason for the blood pressure decrease seen in both clinical and experimental studies after instillation of natural surfactant is an increase in lung volumes after instillation, triggering the lung inflation reflex and resulting in a systemic vasodilation. This would explain why hemodynamic effects have been described mainly after use of rapidly acting porcine surfactant(3–5, 7, 8) and not after instillation of slow acting artificial surfactant(26, 42).
Conclusion. Porcine surfactant causes a systemic vasodilatation and a significant decrease in MABP in newborn lung-lavaged piglets not seen after instillation of the same volume of saline. We speculate that this vasodilatation is caused by activation of the lung inflation reflex. Surfactant transiently increases PVR, but no effects on pulmonary circulation were observed 20 min after instillation.
Abbreviations
- CI:
-
cardiac index
- CO:
-
cardiac output
- CVP:
-
central venous pressure
- Fio2:
-
fraction of inspired oxygen
- FRC:
-
functional residual capacity
- I:E ratio:
-
ratio between inspiration and expiration times
- LAP:
-
left atrial pressure
- MABP:
-
mean arterial blood pressure
- Paco2:
-
partial pressure of arterial CO2
- Pao2:
-
partial pressure of arterial O2
- PAP:
-
mean pulmonary artery pressure
- PEEP:
-
positive end expiratory pressure
- PIP:
-
peak inspiratory pressure
- PVR:
-
pulmonary vascular resistance
- RDS:
-
respiratory distress syndrome
- SVR:
-
systemic vascular resistance
References
Wiseman LR, Bryson HN 1994 Porcine-derived lung surfactant. A review of the therapeutic efficacy and clinical tolerability of a natural surfactant preparation (Curosurf) in neonatal respiratory distress syndrome. Drugs 48: 386–403
Collaborative European Multicenter Study Group 1988 1988 Surfactant replacement therapy for severe neonatal respiratory distress syndrome: an international randomized clinical trial. Pediatrics 82: 683–691
Cowan F, Whitelaw A, Wertheim D, Silverman M 1991 Cerebral blood flow velocity changes after rapid administration of surfactant. Arch Dis Child 66: 1105–1109
Skov L, Hellström-Westas L, Jacobsen T, Greisen G, Svenningsen NW 1992 Acute changes in cerebral oxygenation and cerebral blood volume in preterm infants during surfactant treatment. Neuropediatrics 23: 126–130
Rey M, Segerer H, Kiessling C, Obladen M 1994 Surfactant bolus instillation: effects of different doses on blood pressure and cerebral blood flow velocities. Biol Neonate 66: 16–21
Bell AH, Skov L, Lundstrom KE, Saugstad OD, Greisen G 1994 Cerebral blood flow and plasma hypoxanthine in relation to surfactant treatment. Acta Paediatr 83: 910–914
Skov L, Bell A, Greisen G 1992 Surfactant administration and the cerebral circulation. Biol Neonate 61: 31–36
Segerer H, Vangelder W, Angenent FWM, Vanwoerkens LJPM, Curstedt T, Obladen M, Lachmann B 1993 Pulmonary distribution and efficacy of exogenous surfactant in lung-lavaged rabbits are influenced by the instillation technique. Pediatr Res 34: 490–494
Segerer H, Scheid A, Wagner MH, Lekka M, Obladen M 1996 Rapid tracheal infusion of surfactant versus bolus instillation in rabbits: effects on oxygenation, blood pressure and surfactant distribution. Biol Neonate 69: 119–127
Moen A, Rootwelt T, Robertson B, Curstedt T, Hall C, Saugstad O 1996 Hemodynamics and tissue blood flow after porcine surfactant replacement in surfactant depleted newborn piglets. Pediatr Res 40: 215–224
Raju TNK, Langenberg P 1993 Pulmonary hemorrhage and exogenous surfactant therapy-a metaanalysis. J Pediatr 123: 603–610
Evans NJ, Archer LN 1991 Doppler assessment of pulmonary artery pressure and extrapulmonary shunting in the acute phase of hyaline membrane disease. Arch Dis Child 66: 6–11
Walther FJ, Benders MJ, Leighton JO 1992 Persistent pulmonary hypertension in premature neonates with severe respiratory distress syndrome. Pediatrics 90: 899–904
Seppänen MP, Kääpä PO, Kero PO, Saraste M 1994 Doppler-derived systolic pulmonary artery pressure in acute neonatal respiratory distress syndrome. Pediatrics 93: 769–773
Halliday HL, McCord FB, McClure BG, Reid MM 1989 Acute effects of instillation of surfactant in severe respiratory distress syndrome. Arch Dis Child 64: 13–16
Kääpä P, Seppänen M, Kero P, Saraste M 1993 Pulmonary hemodynamics after synthetic surfactant replacement in neonatal respiratory distress syndrome. J Pediatr 123: 115–119
Hamdan AH, Shaw NJ 1995 Changes in pulmonary artery pressure in infants with respiratory distress syndrome following treatment with Exosurf. Arch Dis Child 72:F176–F179
Bloom MC, Roquesgineste M, Fries F, Lelongtissier MC 1995 Pulmonary haemodynamics after surfactant replacement in severe neonatal respiratory distress syndrome. Arch Dis Child 73:F95–F98
Clyman RI, Jobe A, Heymann M, Ikegami M, Roman C, Payne B, Mauray F 1982 Increased shunt through the patent ductus arteriosus after surfactant replacement therapy. J Pediatr 100: 101–107
Jobe A, Jacobs H, Ikegami M, Jones S 1983 Cardiovascular effects of surfactant suspensions given by tracheal instillation to premature lambs. Pediatr Res 17: 444–448
Vidyasagar D, Maeta H, Raju TN, John E, Bhat R, Go M, Dahiya U, Roberson Y, Yamin A, Narula A, Evans M 1985 Bovine surfactant(surfactant TA) therapy in immature baboons with hyaline membrane disease. Pediatrics 75: 1132–1142
Sanderud J, Norstein J, Saugstad OD 1991 Reactive oxygen metabolites produce pulmonary vasoconstriction in young pigs. Pediatr Res 29: 543–547
Belik J, Light RB 1989 Effect of increased afterload on right ventricular function in newborn pigs. J Appl Physiol 66: 863–869
Haworth S, Hislop A 1981 Adaptation of the pulmonary circulation to extra-uterine life in the pig and its relevance to the human infant. Cardiovasc Res 15: 108–119
Lundstrøm K, Greisen G 1996 Changes in EEG, systemic circulation and blood gas parameters following two or six aliquots of porcine surfactant. Acta Paediatr 85: 708–712
Saliba E, Nashashibi M, Vaillant MC, Nasr C, Laugier J 1994 Instillation rate effects of Exosurf on cerebral and cardiovascular haemodynamics in preterm neonates. Arch Dis Child 71:F174–F178
Chan J, Moglia B, Reeves I, Kim A, Darrow K, Seaton J, Marks K 1994 Cardiorespiratory and stress hormone responses during first dose surfactant administration in neonates with RDS. Pediatr Pulmonol 17: 246–249
Jorch G, Rabe H, Garbe M, Michel E, Gortner L 1989 Acute and protracted effects of intratracheal surfactant application on internal carotid blood flow velocity, blood pressure and carbon dioxide tension in very low birth weight infants. Eur J Pediatr 148: 770–773
Rootwelt T, Loberg EM, Moen A, Oyasaeter S, Saugstad OD 1992 Hypoxemia and reoxygenation with 21% or 100% oxygen in newborn pigs: changes in blood pressure, base deficit, and hypoxanthine and brain morphology. Pediatr Res 32: 107–113
Daly MDB, Hazzledine JL, Ungar A 1967 The reflex effects of alterations in lung volume on systemic vascular resistance in the dog. J Physiol 188: 331–351
Daly MDB, Robinson B 1968 An analysis of the reflex systemic vasodilator response elicited by lung inflation in the dog. J Physiol 195: 387–406
Cheng EY, Kay J, Hoka S, Bosnjak ZJ, Coon RL, Seagard JL, Kampine JP 1989 Influence of lung inflation reflex on vascular capacitance in the systemic circulation. Am J Physiol 256: 1004–1011
Hausdorf G, Hellwege HH 1987 Influence of positive end-expiratory pressure on cardiac performance in premature infants: a Doppler-echocardiographic study. Crit Care Med 15: 661–664
Vilstrup C, Gommers D, Bos JA, Lachmann B, Werner O, Larsson A 1992 Natural surfactant instilled in premature lambs increases lung volume and improves ventilation homogeneity within five minutes. Pediatr Res 32: 595–599
Edberg KE, Sandberg K, Silberberg A, Ekstrom JB, Hjalmarson O 1991 Lung volume, gas mixing, and mechanics of breathing in mechanically ventilated very low birth weight infants with idiopathic respiratory distress syndrome. Pediatr Res 30: 496–500
Goldsmith LS, Greenspan JS, Rubenstein SD, Wolfson MR, Shaffer TH 1991 Immediate improvement in lung volume after exogenous surfactant: alveolar recruitment versus increased distention. J Pediatr 119: 424–428
Cotton RB, Olsson T, Law AB, Parker RA, Lindstrom DP, Silberberg AR, Sundell HW, Sandberg K 1993 The physiologic effects of surfactant treatment on gas exchange in newborn premature infants with hyaline membrane disease. Pediatr Res 34: 495–501
Kelly E, Bryan H, Possmayer F, Frndova H, Bryan C 1993 Compliance of the respiratory system in newborn infants pre- and postsurfactant replacement therapy. Pediatr Pulmonol 15: 225–230
Stenson B, Glover R, Parry G, Wilkie R, Laing I, Tarnow-Mordi W 1994 Static respiratory compliance in the newborn. III. Early changes after exogenous surfactant treatment. Arch Dis Child 70:F19–F24
de Winter J, Merth I, van Bel F, Egberts J, Brand R, Quanjer H 1994 Changes of respiratory system mechanics in ventilated lungs of preterm infants with two different schedules of surfactant treatment. Pediatr Res 35: 541–549
Sindelar R, Dammann V, Jonzon A, Schaller P, Schultze A, Sedin G 1996 Response of slowly adapting pulmonary stretch receptors in surfactant depleted cat lungs: before and after surfactant replacement. Pediatr Res 40: 552( abstr)
van de Bor M, Ma E, Walther F 1991 Cerebral blood flow velocity after surfactant instillation in preterm infants. J Pediatr 118: 285–287
Acknowledgements
The authors acknowledge the technical assistance of Roger Ødegård. We also thank Bengt Robertson and Tore Curstedt for valuable discussions and support.
Author information
Authors and Affiliations
Additional information
Supported by the Norwegian Research Council, Professor Dr. Med. Carl Sembs Fund for Medical Research, and Grants from the Medical Faculty, University of Oslo, Medinnova and Serono Nordic AB.
Rights and permissions
About this article
Cite this article
Moen, A., Yu, XQ., Rootwelt, T. et al. Acute Effects on Systemic and Pulmonary Hemodynamics of Intratracheal Instillation of Porcine Surfactant or Saline in Surfactant-Depleted Newborn Piglets. Pediatr Res 41, 486–492 (1997). https://doi.org/10.1203/00006450-199704000-00006
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199704000-00006
This article is cited by
-
A novel delivery system for supraglottic atomization allows increased lung deposition rates of pulmonary surfactant in newborn piglets
Pediatric Research (2020)
-
Surfactant Therapy for Respiratory Distress Syndrome in Premature Neonates
American Journal of Respiratory Medicine (2002)
-
Oxygen delivery and consumption in surfactant-depleted newborn piglets
Intensive Care Medicine (1998)