Abstract
One of the underlying causes of pathophysiology of meconium aspiration syndrome is access of meconium to the alveolar space and inhibition of activity of lung surfactant. This study examines the effects of meconium on type II cell function by following surfactant secretion. Isolated rat alveolar type II cells were labeled with [methyl-3H]choline during the initial 21-22 h of incubation. During the subsequent 150 min of incubation, phosphatidylcholine (PC) secretion in the presence of 1% meconium was increased 250 ± 11% (mean ± SE, n = 23) over controls. The secretagogue effect was concentration-dependent and reached a maximum at 0.5% meconium. The meconium effect was not due to cellular toxicity as evaluated by vital dye exclusion, lactate dehydrogenase release, and PC synthesis. The secretagogue effect of meconium was associated with the particulate fraction pelleted by centrifugation of the suspension for 1 h at 100,000 × g. Heat treatment of meconium decreased the effect, suggesting the active component to be a protein. The effect of meconium was additive with that of 0.1 mM terbutaline, or 1 mM ATP, suggesting different pathways of action of each agent. The effect of meconium was reduced in the presence of 0.1 mM 4,4′-diisothiocyanato-2,2′-disulfonic acid, or 100 ng/mL surfactant protein A. These agents were previously shown to inhibit surfactant secretion in a stimulus-independent manner. Our results suggest that meconium at low concentrations is not toxic to type II cells, and a component of meconium, possibly a protein, increases PC secretion.
Similar content being viewed by others
Main
Meconium aspiration syndrome is a disease unique to the newborn infant in which meconium stool is passed at some time before birth and aspirated into the lungs before or during parturition. The severity of the disease is variable from only mild respiratory distress to respiratory failure in association with pulmonary hypertension and persistent fetal circulation. The pathophysiology of meconium aspiration syndrome is vaguely understood at best and consists of mechanical obstruction of the airways by the thick adherent meconium, as well an inflammatory reaction and pneumonitis(1). Another contributing factor can be inactivation of lung surfactant, because in vitro studies have shown that meconium decreases the ability of lung surfactant to reduce surface tension(2, 3). This is also supported by recent studies demonstrating the presence of intratracheally instilled meconium in alveolar spaces of newborn rabbits(2). Such presence of meconium in the vicinity of type II cells likely affects their cell function. Bile salts are present in human meconium; in concentrations equal to 10-20% of whole meconium, these salts increase intracellular calcium, and decrease leucine incorporation into cellular protein, suggesting that high concentrations of meconium are toxic to type II cells in culture(4).
PC, the major surface-active component of lung surfactant, is synthesized and secreted by type II cells of alveolar epithelium. The secretion of PC by type II cells can be stimulated by various agents, including agonists forβ-adrenergic receptors, histamine, endothelin-1, vasopressin, and purinoreceptors(5–9). The secondary changes associated with these agonists include increased diacylglycerol, cytosolic calcium, and cellular adenosine 3′,5′-cyclic monophosphate, indicating activation of various protein kinases(5–12). Of various agonists for receptors, ATP appears to act at multiple receptor sites activating a complex series of processes.
This study reports the effect of low concentrations of meconium on the function of isolated alveolar type II cells as evaluated by changes in surfactant secretion. We have also subfractionated meconium by differential centrifugation and chemical solubility, to determine the physical and chemical nature of the active component of meconium.
METHODS
Materials. [methyl-3H]Choline and dipalmatoyl phosphatidyl-[methyl-14C]choline were obtained from Amersham Corp., Arlington Heights, IL. Elastase was obtained from Worthington Biochemical Corp., Freehold, NJ. Tissue culture medium was obtained from Life Technologies, Inc., Grand Island, NY. Plastic tissue culture dishes were obtained from Costar, Cambridge, MA. Rat serum IgG, DNAase, vandate-free ATP, terbutaline, DIDS, and standard chemicals were obtained from Sigma Chemical Co., St. Louis, MO. Staurosporin and H-7 were obtained from Calbiochem, La Jolla, CA.
Purification of SP-A. Bronchoalveolar lavage fluid from bovine lungs obtained from a local slaughter house was processed for purification of lung surfactant by differential centrifugation followed by sodium bromide-sodium chloride gradient centrifugation(13). Purified surfactant was extracted with n-butanol to remove hydrophobic proteins and lipids, extracted in octyl-β-glucoside, after which SP-A was extracted during overnight extraction in Tris buffer(14).
Meconium preparation. The first meconium stool was collected from healthy term newborn infants with no history of complications before, during, or after delivery. There was no documented history of drug use by the mothers before delivery, nor did the babies receive any medication other than erythromycin eye ointment and intramuscular vitamin K. The meconium, without contamination from urine, was collected in disposable diapers by the nursing staff. The diaper was place in a plastic bag, sealed, and kept on ice until received by the investigating team, usually within a few hours. Two pooled samples of meconium from two sets of five healthy babies were used for these studies. A 20% homogeneous slurry of meconium in 0.9% NaCl solution was prepared, divided into 1-mL aliquots in plastic vials, and stored at -80°C for later use to avoid repetitive freeze-thawing of meconium. The dry weight of the 20% meconium slurry from each batch was 43 mg of meconium/mL as determined after lyophilization.
For some experiments, 20% meconium was heated at 100°C for 1 h to denature proteins. The heat-treated meconium was made up to the original volume with water before analyzing its effect on type II cells. For other experiments, meconium was centrifuged at 100,000 × g for 1 h, and the pellet reconstituted in 0.9% NaCl by sonication to the initial volume. Both the supernatant and the reconstituted meconium pellet were used in secretion experiments. For some secretion experiments, meconium was dialyzed for 24 h against sterile 0.9% NaCl using dialysis tubing (Spectro/Por Membranes, Spectrum Medical Industries, Inc., Houston, TX) with a molecular weight cut off of 3500. For another set of experiments, lipids were extracted from 2 mL of 20% meconium in chloroform:methanol according to a previously published method(15). The lipid extract was evaporated to dryness under a stream of N2, and dried lipids were reconstituted by sonication, in a bath sonicator, in 2 mL of 0.9% NaCl. The final concentration in the tissue culture plates in all of the experiments using altered meconium or components of meconium was equal to 1% of original meconium.
Isolation of alveolar type II cells. Pathogen-free male Sprague-Dawley rats (180-200 g of body weight) were used for isolation of alveolar type II cells according to Dobbs et al.(16) as described previously(11). In brief, rats were anesthetized with sodium pentobarbital (50 mg/kg, intraperitoneally), tracheostomized, and exsanguinated. The lungs were cleared of residual blood, by perfusion via the pulmonary artery with solution I containing (in mM) 154 NaCl, 10 glucose, 2.6 phosphate buffer, pH 7.4, 10 HEPES, pH 7.4, 1.7 CaCl2, 1.3 MgSO4, 10 μg/mL streptomycin, and 100 μg/mL penicillin while maintaining ventilation. The lungs were removed, lavaged with solution I and solution II (Ca- and Mg-free solution I plus 0.16 mM EGTA), and treated (three times) intratracheally with an elastase solution (3 U/mL elastase). The lung lobes were minced on a tissue chopper, suspended in solution I containing DNAase, and mixed vigorously for 2 min in a water bath at 37°C. The free cells in suspension were sequentially filtered through 160-, 37-, and 15-μm nylon filters, and centrifuged for 10 min at 300 × g. The pelleted cells were resuspended in MEM and incubated for 1 h at 37°C on 100-mm IgG-coated bacteriologic culture dishes. The free cells were subsequently collected by “panning,” centrifuged, and resuspended in MEM containing 10% fetal bovine serum. The cells were plated on 35-mm tissue culture plates at a density of 1 × 106 cells in 1.5 mL MEM-10% fetal bovine serum. The cells were then incubated for 20-22 h (overnight) at 37°C in humidified air containing 5% CO2. Cells attached to the tissue culture plates after overnight incubation were >92% type II cells as evaluated by staining with phosphine 3R, and >98% of these were viable as judged by the exclusion of the vital dye, erythrocin B.
Evaluation of the toxicity of meconium. Type II cells attached to tissue culture plastic were washed (eight times) with MEM to remove any dead, nonadherent cells, and incubated for 2 h in fresh MEM containing 0, 1, 5, or 10% meconium. At the end of the incubation period, erythrocin B (0.01% final concentration) was added to the medium, and the percentage of cells not excluding dye was determined by viewing under a light microscope. At least 100 cells were counted in each sample. When 5 or 10% meconium was used, the plates had to be washed once with MEM before microscopic examination. Dense meconium solution at these concentrations makes microscopic examination difficult. Thus cell death at these concentrations may be an underestimate.
Cellular toxicity of 1% meconium was also studied by following LDH release into the medium. After overnight incubation, cells were washed (eight times) with MEM, equilibrated for 30 min, and then incubated for 2 h in the absence or presence of 1% meconium. At the end of each incubation, cells and medium were collected for LDH assay by following the oxidation of NADH at 340 nm(17).
To evaluate the effect of meconium on PC synthesis, cells after 20-22 h of incubation were incubated for 3 h in MEM with 100 μM[methyl-3H]choline (specific activity, 5 μCi/μmol) without or with 1% meconium. The cells on plastic dishes were washed three times with MEM and harvested by scraping into 1.5 mL of 0.1% Triton X-100 solution. The lipid extract was dried under N2 and reconstituted in chloroform:methanol (20:1). Lipids were then separated into individual phospholipid classes by thin layer chromatography on boric acid-impregnated Silica Gel G plates(18). Lipids comigrating with authentic PC were scraped and processed for measurement of radioactivity by scintillation counting, or for phospholipid phosphorous assay according to Marinetti(19) as described previously(20).
Effect of meconium on surfactant secretion. For 20-22 h culture type II cells were incubated with 0.5 μCi/mL[methyl-3H]choline to label cellular lipids. After overnight incubation, the attached cells were washed (eight times) with MEM, fresh MEM(1.5 mL) was added, and the cells were equilibrated for 30 min. At the end of the equilibration period, some plates were removed for analysis of labeled lipids in the media and cells. This provided the time 0 secretion. Other culture dishes were further incubated for indicated periods of time without change of the medium. Meconium or its subfractions, or other secretagogues including 1 mM ATP, or 100 μM terbutaline were added to some of the culture dishes at the end of the equilibration period. All inhibitors of PC secretion, including staurosporin (1 μM), H-7 (20 μM), DIDS (100 μM), or SP-A(100 ng/mL medium) were added at the start of the 30-min equilibration period.
At the end of each incubation period the medium was removed and centrifuged for 10 min at 300 × g to remove any floating cells that may have came off during incubation. The cells in the pellet were pooled with those remaining on the tissue culture plate. Routinely, some cells come off during the 2-h incubation period. The supernatant medium and the cells were extracted for lipids after addition of egg PC (400 μg) as a carrier lipid and [14C]DPPC as a tracer lipid. Radioactivity in the lipid extract was quantitated in a scintillation counter, and the results were corrected for recoveries of [14C]DPPC. The secretion was expressed as disintegrations/min in the medium lipids × 100/dpm in the medium plus cell lipids.
In experiments designed to evaluate the role of external calcium, tissue culture plates were washed five times with MEM, and then three times in the incubation buffer containing (in mM) 117.6 NaCl, 35 NaHCO3, 4.7 KCl, 1.2 KH2PO4, 1.2 MgSO4, 30 HEPES, pH 7.4, 10 glucose, with or without 1.3 CaCl2. Cells were equilibrated with 5% CO2 in air, and then incubated for 2 h in the absence or presence of 1% meconium. Subsequent processing was similar to that described above.
All experiments on the synthesis and secretion of PC were conducted in duplicate, and the results were averaged to obtain a single data point for each set of observations. Results are given as mean ± SE. All results were evaluated for statistical significance by t test for paired experiments between two groups, or by one-way analysis of variance for comparison between multiple groups followed by Tukey's post hoc test for significance between any two groups. The differences were considered significant at p < 0.05.
RESULTS
Toxicity. At low concentrations, meconium was not toxic to type II cells in culture. The percentage of cells excluding erythrocin B was similar in the absence or presence of 1% meconium. Fewer cells excluded this dye at higher concentrations of meconium (Fig. 1). It may be pointed out that in 5 and 10% meconium, the dye exclusion rate may be falsely elevated secondary to the need to wash off the meconium to examine plates under the microscope. This, however, was not necessary in experiments with 1% meconium. During 2 h of incubation, the release of LDH in the medium was 0.49 ± 0.1% and 1.90 ± 0.16% (mean ± SE, n= 4, p < 0.05) in the absence or presence of 1% meconium. However, LDH release in these experiments may not be a suitable parameter to determine cellular toxicity. Although meconium itself had a no detectable LDH activity, it stimulated LDH activity when added to the control medium. In two experiments the medium from control cells contained 0.45 ± 0.05% of cell LDH activity, but the activity was 1.68 ± 0.86% when this control medium was assayed in the presence of 1% meconium. This possibly accounts for higher LDH activity in the medium from meconium treated cells.
Meconium effect on viability of type II cells. After overnight culture, type II cells were incubated for 2 h in the absence or presence of indicated concentrations of meconium and assayed for exclusion of the vital dye erythrocin B. In 1% meconium 98.8 ± 0.2% (mean ± SEM, n = 9) cells excluded the vital dye, which was not different from the control cells. Survival rates of 72.0 ± 1.3% (n = 3) in 5% meconium and 53.0 ± 2.9% (n = 3) in 10% meconium were significantly different from control (p < 0.05).
PC Synthesis. Synthesis of PC as measured by the incorporation of [methyl-3H]choline into cellular PC was unaltered in the presence of 1% meconium. In experiments with four separate cell preparations, incorporation of choline into PC during 3 h of incubation was 2.32 ± 0.68 and 3.00 ± 0.90 nmol/μg PC phosphorus (mean ± SE,p > 0.05) in the absence or presence of 1% meconium.
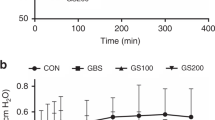
PC Secretion. Compared with control cells, the secretion of PC was higher in the presence of 1% meconium. During 2 h of incubation, PC secretion in the presence of meconium was increased by 250 ± 11% (mean± SEM, n = 23, p < 0.001) over that in the absence of meconium. Meconium increased PC secretion in a dose-dependent manner (Fig. 2). The apparent EC50 (concentration to elicit 50% of maximal effect) was 0.25%.
Next, we investigated whether lipids or proteins of meconium acted as surfactant secretagogues. A 1-h treatment of meconium at 100°C decreased the secretagogue effect of meconium significantly, suggesting that a protein component contributed to the increase in PC secretion. This was also supported when the secretagogue effect of the lipid-soluble components of meconium was investigated. Compared with whole meconium, the lipid extract of meconium had significantly reduced effect on PC secretion (Fig. 3).
The effect of heat-treated meconium and subfractions of meconium on PC secretion. Results are expressed as the mean ± SEM. The number in parentheses represents the number of experiments. *p < 0.05 vs meconium or meconium pellet. **p < 0.05vs heat-treated meconium, lipid extract of meconium, or meconium supernatant.
Meconium was separated into the supernatant and pellet fractions after differential centrifugation of 20% slurry of meconium. The supernatant did not increase PC secretion over the control. The resuspended pellet at a concentration equal to 1% meconium, however, increased PC secretion by 2.6± 0.51-fold over that of the control (Fig. 3). Thus, the active component of meconium was associated with large particles in meconium. Similarly, the effect of dialyzed meconium was not different from that of whole meconium, supporting the hypothesis that the active component is in the large particulate fraction.
In an attempt to understand the mechanism of the secretagogue effect of meconium, the secretion of PC was measured in the presence of meconium and in the absence or presence of other known secretagogues of surfactant. In these experiments, we used the maximum stimulatory concentration of these agents(21). In case the mechanism of secretagogue effect of meconium is not shared by either of these agents, then the effect of meconium and these agents on surfactant secretion should be additive. The secretion of PC in the presence of terbutaline, a β-agonist was 2.19 ± 0.09%, with a control of 1.64 ± 0.19%, whereas the secretion in the presence of 1% meconium was 4.33 ± 0.61%. The effect of meconium in the presence of terbutaline was not significantly different from the calculated sum of the individual effects of meconium and terbutaline on PC secretion (4.45 ± 0.62% versus 4.89 ± 0.48%, n = 4), suggesting they function to increase PC secretion through different mechanisms. Similarly, the effect of meconium on PC secretion was additive with ATP, an agonist for purinoceptors. In these experiments, 1% meconium-induced PC secretion was 4.31± 37% and 1 mM ATP-induced secretion was 4.40 ± 0.58%(n = 6). The combined effects of ATP and meconium (6.19 ± 0.53%) was not different from the calculated sum (6.76 ± 0.73%) of the individual effects of, these agents, suggesting that they do not share a common pathway in inducing surfactant secretion.
The percentage increase in PC secretion over control in the presence of 1% meconium was similar in cells incubated in calcium-free buffer or in cells incubated in calcium-containing buffer (202 ± 16% versus. 262± 17%, n = 6). This suggests that meconium-induced PC secretion does not work through a calcium-dependent mechanism(Table 1). Further experiments to investigate the mechanism of meconium effect showed that the secretagogue action was unlikely by activation of protein kinase C, because protein kinase C inhibitors, H-7 or staurosporin, did not block meconium-stimulated PC secretion(Table 2). PC secretion in the presence of 1% meconium and 100 μM DIDS was significantly less than with 1% meconium alone(Table 2). DIDS is known to block PC secretion distal to generation of second messenger and possibly at the plasma membrane level(11). Another inhibitor of surfactant secretion that acts distal to generation of second messenger is SP-A. SP-A (100 ng/mL) also significantly decreased the meconium induced increase in PC secretion(Table 2).
DISCUSSION
Meconium, if aspirated in the perinatal period, is associated with severe lung disease, including pneumonitis and pulmonary hypertension. Recently meconium has been shown to decrease the surface-active properties of surfactant(2, 3). Previous studies have indicated cellular toxicity of meconium at high concentrations(4). Using alveolar type II cells in culture, this is the first report demonstrating that meconium can alter type II cell function at low concentrations. The clinical significance of this observation is manifest in the altered cellular function with respect to surfactant metabolism, rather than just the physical inactivation of alveolar surfactant.
Although meconium at >1% concentrations was toxic to cultured type II cells (LD50, ≈10%) as evaluated by a decrease in vital dye exclusion, low concentrations of meconium were not toxic to these cells. The cellular toxicity of 1% meconium was evaluated by three different methods, changes in vital dye exclusion, LDH activity, and PC synthesis. Meconium decreased neither the exclusion of erythrocin B, nor the synthesis of PC in these cells. Cellular toxicity should be associated with a decrease in the synthesis of PC. Although the LDH activity was increased in the medium of cells incubated with meconium, LDH activity also increased when meconium was added to the medium recovered after incubation of control cells. Thus, evaluating LDH release may not be appropriate to determine cellular toxicity of meconium.
The component of meconium that stimulates surfactant PC secretion is associated with the large particulate fraction. Based on the observation that heating meconium results in loss of its secretagogue effect, we speculate that the active component is a protein. This belief is also supported by the observation that the lipid-soluble component of meconium had no effect on surfactant secretion. Previous studies have reported that the effects of meconium on surfactant function and on cell viability were due to lipid-soluble components, such as fatty acids and bile salts(2, 4). Thus, both the lipid and protein components of meconium affect surfactant function and metabolism.
The mechanism of the secretagogue effect of meconium is not clear. When maximal stimulatory concentrations of ATP or terbutaline were studied in the absence or presence of meconium the secretion with meconium was additive with that of ATP or terbutaline. Such observations suggest that meconium acts through a mechanism that is not shared by either of these agents. Terbutaline and ATP work through second messengers, diacylglycerol and cAMP, to activate various protein kinases(5, 9, 10, 21). Supporting this conclusion is the observation that the meconium effect could not be blocked by protein kinase C inhibitors, H-7 and staurosporin. The secretagogue effect of meconium could, however, be partially blocked by SP-A and DIDS which act distal to the second messenger. Calcium does not appear to play a major role in meconium-induced PC secretion as the percent increase of PC secretion over controls was not significantly less in experiments using calcium-free medium.
The possibility that the effect of meconium could be due to a metabolite of a drug or medication taken by the mother is unlikely as there was no documented drug use in the history of the mothers. Also, the infants, from whom the meconium was collected, received no medication other than erythromycin eye ointment and intramuscular vitamin K. Additionally, dialysis of meconium to remove possible drug metabolites did not decrease its secretagogue effect.
In summary, meconium appears to have an effect on type II cell function in vitro by stimulating surfactant secretion. We postulate that a protein component of meconium is responsible for this effect. We were unable to clearly define a pathway through which meconium acts to increase surfactant secretion. Although at this point we cannot rule out that secretagogue effect is a manifestation of a subtle toxicity of meconium, our observation that the effect could be blocked with two structurally different agents, SP-A, and DIDS, would argue against such a possibility. Future research will be directed at isolating the component in meconium that is active in stimulating surfactant secretion as well as delineating the pathway through which it acts to increase surfactant secretion.
Abbreviations
- PC:
-
phosphatidylcholine
- DIDS:
-
4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid
- SP-A:
-
surfactant protein A
- MEM:
-
Eagle's minimum essential medium
- LDH:
-
lactate dehydrogenase
- ED50:
-
dose required to cause 50% effect
- LD50:
-
dose required to cause 50% death
- HEPES:
-
N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid
References
Lopez A, Bildfell R 1992 Pulmonary inflammation associated with aspirated meconium and epithelial cells in calves. Vet Pathol 29: 104–111.
Sun B, Cursted T, Robertson B 1993 Surfactant inhibition in experimental meconium aspiration. Acta Paediatr 82: 182–189.
Moses D, Holm BA, Spitale P, Liu MY, Enhorning G 1991 Inhibition of pulmonary surfactant function by meconium. Am J Obstet Gynecol 164: 477–481.
Oelburg DG, Downey SA, Flynn MM 1990 Bile Salt induced intracellular Ca++ accumulation in type II pneumocytes. Lung 168: 297–308.
Brown LA, Longmore WJ 1981 Adrenergic and cholinergic regulation of lung surfactant secretion in the isolated perfused lung and in the alveolar type II cell in culture. J Biol Chem 256: 66–72.
Chen M, Brown LAS 1990 Histamine stimulation of surfactant secretion from rat type II peumocytes. Am J Physiol 258:L195–L200.
Sen N, Grunstein M, Chander A 1994 Stimulation of lung surfactant secretion by endothelin-1 from rat alveolar type II cells. Am J Physiol 266:L255–L262.
Brown LA, Chen M 1990 Vassopressin signal transduction in rat type II pneumocytes. Am J Physiol 258:L301–L307.
Rice WR, Dorn CC, Singlton M 1990 P2-purinoceptor regulation of surfactant phosphatidylcholine secretion. Relative roles of calcium and protein kinase C. Biochem J 266: 407–413.
Sen N, Chander A 1994 Alkalosis- and ATP-induced increases in the diacylglycerol pool in alveolar type II cells are derived from phosphatidylcholine and phosphatidylinositol. Biochem J 298: 681–687.
Chander A, Sen N 1993 Inhibition of phosphatidylcholine secretion by stilbene disulfonate in alveolar type II cells. Biochem Pharmacol 45: 1905–1912.
Corbet A, Voelker R, Murphy F, Owens M 1991 Effect of calcium and calcium antagonists on phospholipid secretion induced by lung inflation in newborn rabbits. Am J Med Sci 301: 102–114.
Katylal SL, Esters LW, Lombardi B 1977 Method for the isolation of surfactant from homogenates and lavages of adult, newborn, and fetal rats. Lab Invest 36: 585–592.
Wright JR, Wager RE, Hawgood S, Dobbs L, Clements JA 1987 Surfactant apoprotein Mr = 26,000-36,000 enhances uptake of liposomes by type II cells. J Biol Chem 262: 2888–2894.
Bligh EG, Dyer WJ 1959 A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917.
Dobbs LG, Gonzales R, Williams M 1988 An improved method for isolating type II cells in high purity and yield. Am Rev Respir Dis 134: 141–145.
Lee YP, Lardy HA 1965 Influence of thyroid hormones on L-α-glycerophosphate dehydrogenase in various organs of rat. J Biol Chem 240: 1427–1436.
Fine JB, Sprecher H 1982 Unidimensional thin-layer chromatography of phospholipids on boric acid impregnated plates. J Lipid Res 23: 660–663.
Marinetti GV 1962 Chomatographic separation, identification, and analysis of phosphatides. J Lipid Res 3: 1–20.
Chander A, Fisher AB, Strauss JF 1982 Role of an acidic compartment in synthesis of disaturated phosphatidylcholine by rat granular pneumocytes. Biochem J 208: 651–658.
Chander A, Sen N, Wu A, Spitzer AR 1995 Protein kinase C in ATP regulation of lung surfactant secretion in type II cells. Am J Physiol 268:L108–L116.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Higgins, S., Wu, AM., Sen, N. et al. Meconium Increases Surfactant Secretion in Isolated Rat Alveolar Type II Cells. Pediatr Res 39, 443–447 (1996). https://doi.org/10.1203/00006450-199603000-00011
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199603000-00011
This article is cited by
-
Pulmonary surfactant kinetics of the newborn infant: novel insights from studies with stable isotopes
Journal of Perinatology (2009)
-
What’s new in surfactant?
European Journal of Pediatrics (2007)
-
Meconium aspiration syndrome: Current concepts and management
Comprehensive Therapy (1999)