Abstract
Vascular permeability factor (VPF) is the most potent known mediator of vessel wall permeability. In the kidney, it is expressed preferentially in the glomerular visceral epithelial cells. The present study was designed to clarify the proposed role of VPF in diseases with increased glomerular permeability as here exemplified by the congenital nephrotic syndrome of the Finnish type (CNF). For this, we studied the expression levels and the sites of synthesis of VPF and its kinase-insert domain receptor (KDR) in kidneys of patients with CNF using Northern and in situ hybridization techniques and immunohistologic staining with anti-VPF antibody. In addition, we extended the study to include analysis of fetal kidney tissue and cultured glomerular cells of normal and CNF kidneys. In CNF and in normal kidneys VPF was localized in the visceral epithelial aspect of the glomeruli and in the collecting ducts, as also earlier described. A new finding was its localization also in the juxtaglomerular area. The VPF receptor KDR was found in glomeruli in the endothelial cells, but it was not detected in the peritubular capillaries. No consistent differences in the levels of VPF or KDR mRNAs or in their sites of production were seen in CNF and control samples. Also the distribution of VPF antigen in the CNF kidneys and normal kidneys was similar. Thus, we propose that VPF and KDR are not directly involved in the pathogenesis of the proteinuria in CNF.
Similar content being viewed by others
Main
The glomerular capillary wall is a complex filter composed of highly differentiated endothelial and epithelial cells resting on a glomerular basement membrane(1). Despite the high hydraulic conductivity (150 L/d), the glomerular capillary wall imposes an efficient barrier to the passage of plasma proteins the size of albumin and larger(2). Glomerular permeability to circulating macromolecules has been extensively studied in the last few years with the aim of elucidating the mechanisms by which the glomeruli lose their filtration function during many glomerular diseases. Reduced glomerular permselectivity, then, leads to the nephrotic syndrome with proteinuria, hypoalbuminemia, and edema(3). Experimental models of glomerular diseases have been widely used to show that circulating macromolecules are retained within the lumen of glomerular capillaries based on their size, shape, and electric charge(4–6). However, the functional nature of the glomerular filtration barrier and the factors that determine size and charge selectivity are still not well defined.
The VPF is the most potent known mediator of vessel wall permeability. It was first described in the conditioned media of several rodent and human tumor cell lines(7, 8). VPF is also known as VEGF, as it stimulates endothelial cell proliferation in cell culture conditions and angiogenesis in vivo, especially during tissue maturation(9). The mitogenic activity of VPF appears to be mediated by specific receptors, which are found exclusively on the surface of endothelial cells(10–12). Recent studies have identified a growing family of receptor tyrosine kinase genes, the currently known ones including KDR(13) and FLT-1(14), encoding the human cell-surface receptors for VPF.
VPF is active at the level of a few nanograms and, on a molar basis, increases microvascular permeability with a potency some 50 000 times that of histamine(15). By alternative splicing of its mRNA, VPF exists in four different homodimeric molecular species, each monomer having 121, 165, 189, or 206 amino acids, respectively(16–18). VPF121 and VPF165 are soluble and secreted proteins, whereas VPF189 and VPF206 are bound to heparin-containing proteoglycans of the cell surface or of the basement membrane(19). The mechanism of VPF-induced vascular permeability is as yet incompletely understood. However, VPF does not act by releasing second mediators of permeability such as histamine, kinins, or products of arachidonic acid metabolism(7). Instead, the data of Brock et al.(20) indicate that VPF acts directly on endothelial cells, causing a rapid phospholipase C activation and intracellular Ca2+ increase.
Because VPF is an extremely potent agent in promoting fluid and protein extravasation, it is a particularly interesting mediator in many functions in normal and pathologic physiology. VPF is proposed to modulate the integrity of the blood-brain barrier and regulate the transport of small molecules across brain capillaries and pulmonary alveoli(21). Moreover, VPF is supposed to participate in processes known to involve changes in vascular permeability during inflammation, wound healing, and tumor-associated vascular leakiness(22–25).
Glomerular permeability to circulating macromolecules has been extensively studied to elucidate the mechanisms by which the normal glomerular permeability barrier is obtained during maturation, maintained in normally functioning kidneys, and lost in diseases with proteinuria. Thus, the recent finding of Brown et al.(26) of VPF localization preferentially in the glomerular visceral epithelium was of great interest and suggested the possibility that VPF could be involved in the glomerular permeability changes. In this study, we explored the putative involvement of VPF and its receptor KDR in the pathogenesis of CNF, a renal disease characterized by massive, treatment-resistant proteinuria starting already in utero(27–29). For this, the expression levels of VPF and KDR and the cytologic sites of their production were studied in the kidneys and cultured glomerular cells of patients and control subjects.
METHODS
Tissue samples. As normal human kidney tissues we used cadaver kidneys unsuitable for renal transplantation due to vascular anatomic reasons(n = 3; IV Department of Surgery, University of Helsinki, Finland) or macroscopically normal parts of kidneys obtained at nephrectomy due to renal carcinoma (n = 3; II Department of Surgery, University of Helsinki). A fetal kidney sample was from a legal, prostaglandin-induced abortion at the 16th week of gestation due to a severe maternal illness (I Department of Obstetrics and Gynecology, University of Helsinki). Kidney samples from patients with CNF were obtained at therapeutic nephrectomies performed on the affected children at the age of 9-18 mo according to the established treatment protocol (n = 7; Children's Hospital, University of Helsinki)(30). All procedures used in this study were approved by the Ethical Committees of the respective departments of the University Central Hospital.
Immediately after removal of the kidneys, the cortical tissues were separated from medulla and dissected to cubes of about 1 cm3. The samples were then prepared for glomerular cell cultures (see below), or snap frozen in isopentane cooled in liquid nitrogen and stored at -70 °C until used for the isolation of RNA or for the preparation of frozen tissue sections.
Isolation of glomeruli and establishment of glomerular cell cultures. Glomeruli were isolated from the kidney samples as described previously(31, 32). Aseptically isolated cortical tissue was placed into ice-cold PBS (pH 7.4), finely minced with a razor blade, and pushed with a spatulum sequentially through sieves of 250- and 150-μm pore sizes. The isolated glomeruli were collected by rinsing from a third sieve of 106-μm pore size. The preparation thus obtained was examined under light microscopy for purity; usually 94-96% pure glomeruli were obtained. For culture, the glomeruli were then placed in 25-cm2 plastic tissue culture bottles (Greiner Labortechnik, Frickenhausen, Germany) in RPMI 1640 medium (GIBCO Biocult, Paisley, Scotland) supplemented with penicillin(100 U/mL; NordCell, Skärholmen, Sweden), streptomycin (100 μg/mL; NordCell), glutamine (2 mM; NordCell), and 10% FCS (Collaborative Research, Cambridge, MA). The medium was changed twice a week after the proliferating cells appeared, typically by d 5 of culture. The outgrowing cell types were identified by their morphology and the expression of cell type-specific epitopes studied by immunofluorescence microscopy(32–35). Briefly, for mesangial cells these included antibodies against Thy 1.1 (at a dilution of 1:40 in PBS; clone OX-7, Serotec, Oxford, England), α smooth muscle actin (1:50, Progen Biotechnik GmbH, Heidelberg, Germany), extracellular domain of fibronectin(1:50, Locus Genex, Helsinki, Finland), integrin α1 (1:50, Becton-Dickinson, San Jose, CA) and α5 (1:50, Telios Pharmaceuticals, San Diego, CA), and Ricinus communis agglutinin(RCA I, 1:20, Vector Laboratories Inc., Burlingame, CA). For epithelial markers we used anti-PHM5 (1:50, Dakopatts, Glostrup, Denmark) and anti-gp44(kindly provided by Dr. Peter Mundel, University of Heidelberg) antibodies, and wheat germ agglutinin (1:20, Vector Laboratories Inc.). The cells used in the present study (passages 3-4) contained both epithelial and mesangial cells, with a predominance (approximately 70%) of mesangial cells.
The adenovirus-transformed human embryonic kidney epithelial cell line A293(American Type Culture Collection, Rockville, MD; ATCC no. CRL 1573) was cultured under the same conditions as for glomerular cells.
RNA isolation and Northern analysis. The guanidine isothiocyanate-cesium chloride method(36) was used to isolate total RNA from tissue samples of 300-500 mg or from about 107 cells harvested from subconfluent cultures. Thirty micrograms of total RNA or 3 μg of poly(A)+ RNA prepared by oligo(dT) cellulose chromatography(Boehringer Mannheim Biochemica, Germany) were electrophoresed in 0.8% agarose gels containing 2.2 M formaldehyde and transferred to nylon membranes (Pall Biodyne, New York, NY)(37). Prehybridization and hybridization were performed according to manufacturer's suggestions with the temperature of 42 °C. The cDNA probes for human VPF (covering a common 580-bp region for VPF165, VPF189, and VPF206 and having a 132-bp insertion with respect to VPF121(16); kindly provided by Dr. Daniel Connolly, Monsato Co., St. Louis, MO) and for its receptor KDR (kindly provided by Dr. Bruce Terman, Lederle Laboratories, Pearl River, NY)(13) were labeled with[α-32P]dCTP (>3000 Ci/mmol; Amersham Corp., UK) by random priming method(38) using a random primer DNA labeling kit (Boehringer). To control the total RNA content and lack of degradation in the analyzed preparations, the blots were rehybridized with a humanβ-actin probe. For autoradiography, the filters were exposed on medical x-ray film (Fuji Photo Film Co., Japan) at -70 °C with intensifying screens.
Immunohistology. Frozen sections 3-4 μm thick were cut from the cortical kidneys and fixed for 3 min in acetone at -20 °C. After repeated washes in PBS, the sections were incubated first with rabbit polyclonal anti-VPF-peptide antibodies recognizing the common N terminus of human VPF variants (5 μg/mL in PBS; VEGF-A20, Santa Cruz Biotechnology Inc., CA) overnight at 4 °C, washed with PBS, and then incubated with rat anti-rabbit IgG antibodies (8 μg/mL in PBS; Zymed, San Francisco, CA) conjugated with FITC. Immunofluorescence microscopy and photography were performed as previously described(33, 35).
In situ hybridization. VPF cRNA probes were synthesized fromHind III (antisense) or EcoRI (sense) linearized pGem3Zf(+) plasmid containing nucleotides 57-638 of the VPF165 coding region(recognizing all four splicing variants, see “RNA isolation and Northern analysis”), using T7 and SP6 polymerases (Promega Corp., Madison, WI) and 35S-UTP (Amersham Corp., UK)(37). For KDR probes, the fragment covering bp 6-715 cloned into pBluescript KS (see“RNA isolation and Northern analysis”) was linearized withEco RI (antisense) or HindIII (sense) and transcribed correspondingly.
Frozen sections 3-4 μm thick were cut from the tissue samples on glass slides pretreated with 3′-(triethoxysilyl)propylamine (Sigma Chemical Co. Co., St. Louis, MO). Prehybridization and hybridization were performed according to Wilcox's(39) procedure for frozen sections. Posthybridization washes were as follows: 1) 5 × SSC (1× SSC: 150 mM NaCl, 15 mM Na-citrate) and 10 mM DTT (Calbiochem Co., Behring Diagnostics, La Jolla, CA) for 30 min at 50 °C; 2) 50% formamide, 2 × SSC, and 30 mM DTT for 30 min at 65 °C;3-5) 0.5 M NaCl, 10 mM Tris-HCl, pH 8, and 5 mM EDTA (NTE) for 3× 10 min at 37 °C; 6) 20 μg/mL RNase A (Sigma Chemical Co.) in NTE for 30 min at 37 °C; 7) NTE for 15 min at 37 °C;8) same as 2); 9), 2 × SSC for 15 min at 65 °C; and 10) 0.1 × SSC for 15 min at 65 °C. After dehydration with increasing concentrations of ethanol and air drying, the sections were covered with NTB-2 photoemulsion (Eastman Kodak Co., Rochester, NY), exposed for 2 wk at 4 °C, developed by the protocol of Kodak, and counterstained with hematoxylin.
RESULTS
Expression levels of VPF and KDR mRNA. The Northern blots containing total or polyadenylated RNA preparations from normal adult, fetal, or CNF kidneys, and the respective cultured glomerular cells were studied for the expression of VPF and KDR mRNAs (Fig. 1). An abundant expression of VPF mRNA was observed in all kidney tissues and in cultured kidney cells. Fetal kidney seemed to express the highest level of VPF mRNA. The major VPF transcript in all samples was detected at ≈3.8 kb, compared with the locations of 18 and 28 S ribosomal RNAs. Additional faint bands of approximately 1.9, 4.5, and 6.5 kb were also seen on longer exposures with poly(A+) RNA samples.
Northern blot analysis of VPF and KDR mRNAs in CNF, in normal kidney tissues, and in cultured renal cells. The samples consist of 30μg of total cellular RNA extracted from human fetal (lane 1), normal adult(lane 2), or CNF (lane 4) kidney cortex, and from cultured glomerular cells of normal (lane 6) and CNF (lane 7) kidneys. Lane 8 demonstrates RNA extracted from the fetal renal epithelial cell line A293. Lanes 3 and 5 show poly(A+) RNA fractions (3 μg/lane) of normal adult or CNF patient kidney cortex, respectively. Hybridization with β-actin probe is shown for a loading control.
The KDR probe detected a single mRNA of 7.0 kb. The most abundant KDR expression was seen in the samples of normal adult and CNF kidneys. A slightly weaker KDR-specific band was seen in fetal kidney. No KDR signals were obtained from cultured kidney cells.
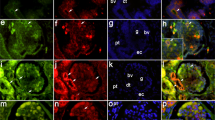
Localization of VPF and KDR mRNA. To obtain further information of the cellular localization of VPF and KDR production in normal and CNF kidneys, we performed in situ hybridization experiments on frozen tissue sections. In accordance with previous reports(26), glomeruli labeled strongly for VPF mRNA in a fashion suggestive of the labeling of podocytes (Fig. 2,A-C). No significant differences were observed in the amount or localization of the silver grains between normal and CNF cortical kidney samples. Rare tubular profiles, identified as collecting ducts based on their morphology, were also labeled (Fig. 2,D and E).
Micrographs demonstrating in situ hybridization of cortical kidney tissues of CNF patients with VPF and KDR probes. VPF antisense riboprobe labeling of glomeruli (g) as visualized by bright-field (A and C) and dark-field(B) microscopy, and of the collecting duct system by bright field(D) and dark field (E). Distinct labeling pattern of glomeruli for KDR mRNA by bright field (F) and dark field(G), correspondingly. Bar represents 50 μm.
A distinct pattern of KDR expression was seen more centrally in the glomerular tuft resembling the pattern of glomerular endothelial reactivity(Fig. 2, F and G), but no KDR-specific signal was seen in the peritubular capillaries. The KDR labeling pattern in the CNF kidneys was comparable to that of the normal kidney. In all samples, the background reactivity level was low, and no specific cellular labeling was seen with the control sense probes.
Localization of VPF protein. We wanted to extend the study also to detect the VPF protein product. Immunohistochemical staining with affinity-purified antibodies against the amino terminus of human VPF confirmed the visceral epithelial localization of VPF in normal kidney glomeruli and in collecting ducts (results not shown). In the samples of CNF kidneys a similar, preferentially visceral epithelium-specific staining could be seen constantly in the glomeruli (Fig. 3A). With some sections intense juxtaglomerular staining also could be seen (Fig. 3B). In the fetal kidney, both the epithelium of the invading ureter and collecting duct (Fig. 3C), as well as the developing glomeruli(Fig. 3D), showed VPF expression. No specific staining was seen with conjugate only.
Immunofluorescence micrographs demonstrating the distribution of VPF protein. Frozen sections from CNF (A), normal adult (B), or fetal (C and D) cortical kidneys were stained with anti-VPF-peptide antibodies, and FITC-conjugated anti-rabbit IgG. In the well peseserved CNF glomerulus (A) most staining is apparently present in podocytes. Also juxtaglomerular cells were positive, as seen in the normal kidney (B). Fetal kidney collecting ducts(C) and the developing glomeruli (D) contained VPF antigen. Bar represents 50 μm.
DISCUSSION
VPF is a potent agent inducing and enhancing microvascular permeability as demonstrated experimentally in guinea pig skin and peritoneal wall permeability assays(7). It is also a bioactive product of various solid tumors, where VPF is proposed to maintain the basal permeability of microvascular endothelia essential for the exchange of nutrients and plasma proteins into the perivascular tissue(7, 22, 25). VPF is abundantly expressed in the glomerular podocytes, and it has been speculated that VPF is involved in the regulation of glomerular permeability(26, 40, 41). Here we have studied the expression of VPF and its receptor KDR in the kidneys of patients with the CNF. CNF is a recessively inherited renal disease in which the massive, treatment-resistant proteinuria commences in utero(27–29) and thus offers an ideal target to test the role of VPF in glomerular permeability.
The genetic defect of CNF has been localized to chromosomal region 19q13.1, but at the present, this region does not contain any known candidate genes for CNF(42), and the basic defect of CNF remains unclear. The success of transplantation therapy without manifestation of new symptoms from any other organs after a 7-8 y follow-up period(30, 43) strongly suggests that the clinical effect of the CNF gene involves the maintenance of glomerular filtration barrier. Morphologically the typical glomerular findings in the advanced states of CNF are sclerosis, mesangial hypercellularity, and retraction of the podocyte foot processes. Retraction and the possible denudation of the glomerular basement membrane reflect a direct damage to podocytes(44). It has been speculated that injury to podocytes, the cytologic site of VPF production, may lead to changes in VPF synthesis and/or secretion and thereby to abnormal glomerular permeability(26). Thus, VPF has been among the candidate molecules proposed to be involved in the pathogenesis of proteinuria. Thus far, there are no data available of the association of VPF in human glomerular diseases presenting with proteinuria, and the present study failed to show any significant differences in the expression of VPF or its receptor KDR in CNF kidneys, but revealed VPF production in previously unrecognized sites of the nephron important for fluid homeostasis. On the basis of the present results, however, the possibility that other mechanisms for proteinuria involving also VPF in other human diseases cannot be excluded. Whether other members of the growing VPF receptor family are involved in CNF also cannot be conclusively ruled out.
In extension to previous findings, we could show here that VPF is expressed in the juxtaglomerular area and in the collecting duct epithelium. Similarly, Simon et al.(45) have noted VPF reactivity at the collecting duct area. Northern blotting analysis revealed that cultured glomerular cells, especially mesangial cells as decided by their morphology and the expression of cell type-specific antigens, express VPF mRNA. Thus, even if the podocytes may be the main site of VPF production in intact glomeruli, this result indicates that mesangial cells are also a potential source of VPF production in vivo, as suggested previously by IIjimaet al.(41). Immunoelectron microscopic studies should be performed, however, to confirm this finding. The observed VPF expression in the epithelial fetal kidney cell line A293 is in accordance with our results showing abundant VPF protein in the epithelial structures of fetal kidney tissue, in the developing glomeruli, and in the epithelium of the ureter and collecting duct.
The finding that VPF is also expressed in juxtaglomerular area and collecting duct epithelium is interesting as these sites are crucially involved in the homeostasis of fluid balance of the body. However, KDR could not be readily detected at these sites, but this may be explained by the relatively low resolution power of in situ hybridization technique. Alternatively, other members of the family of receptors for VPF may be involved. Taken together, the abundance of VPF in juxtaglomerular areas and in collecting duct epithelia deserves further studies, particularly the possible functions of VPF in water channel regulation.
The present study extends the previous findings also in showing that the VPF receptor KDR is found in adult glomeruli apparently in the endothelial cells. Due to the low resolution power of the techniques used and the close proximity of endothelial and mesangial elements in glomeruli, the precise localization of KDR mRNA to endothelial cells could not be unequivocally determined. However, the Northern blot analysis of mesangial cell cultures without detectable KDR strongly suggests an exclusively endothelial expression of KDR in kidney glomeruli and is, furthermore, well in agreement with the published results of VPF receptors in the vascular endothelium(10–12).
In contrast to its transient expression reported in many fetal tissues during development and maturation(46), VPF expression persists in adult kidney, but is reduced into the glomerular visceral epithelium. Interestingly, a constitutive VPF expression has also been reported in the epithelium of the choroid plexus, where the endothelium is similarly highly fenestrated and involved in filtration of the cerebrospinal fluid(47). These findings suggest that VPF may be important for the differentiation and maintenance of organotypic endothelial cells with specific functions. The other known action of VPF in inducing endothelial proliferation may not be its function in glomeruli, because proliferation of glomerular endothelial cells is seldom seen in the adult kidney. Moreover, because we failed to show appreciable changes in the VPF level or cytologic site of production in CNF kidneys, we propose that VPF is not involved in the pathogenesis of proteinuria in this disease.
Abbreviations
- VPF:
-
vascular permeability factor
- CNF:
-
congenital nephrotic syndrome of the Finnish type
- KDR:
-
kinase-insert domain receptor
- VEGF:
-
vascular endothelial growth factor
References
Tisher CC, Madsen KM 1991 Anatomy of the kidney. In: Brenner BM, Rector FC (eds) The Kidney. WB Saunders, Philadelphia, pp 3–75.
Remuzzi A, Remuzzi G 1994 Glomerular perm-selective function. Kidney Int 45: 398–402.
Myers BD, Guasch A 1993 Selectivity of the glomerular filtration barrier in healthy and nephrotic humans. Am J Nephrol 13: 311–317.
Chang R, Ueki I, Troy J, Deen W, Robertson C, Brenner B 1975 Permselectivity of the glomerular capillary wall to macromolecules: experimental studies in rats using neutral dextran. Biophys J 15: 887–906.
Bohrer M, Deen W, Robertson C, Troy J, Brenner B 1979 Influence of molecular configuration on the passage of molecules across the glomerular capillary wall. J Gen Physiol 74: 583–593.
Deen W, Bohrer M, Brenner B 1979 Macromolecules transport across glomerular capillaries: application of pore theory. Kidney Int 16: 353–365.
Senger DR, Galli SJ, Dvorak AM, Peruzzi CA, Harvey VS, Dvorak HF 1983 Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science 219: 983–985.
Senger DR, Peruzzi CA, Feder J, Dvorak HF 1986 A highly conserved vascular permeability factor secreted by a variety of human and rodent cell lines. Cancer Res 46: 5629–5632.
Connolly DT, Heuvelman DM, Nelson R, Olander JV, Eppley BL, Delfino JJ, Siegel NR, Leimgruber RM, Feder J 1989 Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest 84: 1470–1478.
Jakeman LB, Winer J, Bennett GL, Altar CA, Ferrara N 1992 Binding sites for vascular endothelial growth factor are localized on endothelial cells in adult rat tissues. J Clin Invest 89: 244–253.
Quinn TP, Peters KG, De Vries C, Ferrara N, Williams LT 1993 Fetal liver kinase 1 is a receptor for vascular endothelial growth factor and is selectively expressed in vascular endothelium. Proc Natl Acad Sci USA 90: 7533–7537.
Barleon B, Hauser S, Schöllmann C, Weindel K, Marmé D, Yayon A, Weich HA 1994 Differential expression of the two VEGF receptors flt and KDR in placenta and vascular endothelial cells. J Cell Biochem 54: 56–66.
Terman BI, Dougher-Vermazen M, Carrion ME, Dimitrov D, Armellino DC, Gospodarowicz D, Bohlen P 1992 Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem Biophys Res Commun 187: 1579–1586.
De Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT 1992 The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science 255: 989–991.
Senger DR, Connolly D, Van De Water L, Feder J, Dvorak HF 1990 Purification and NH2-terminal amino acid sequence of guinea pig tumor-secreted vascular permeability factor. Cancer Res 50: 1774–1778.
Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N 1989 Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246: 1306–1309.
Houck KA, Ferrara N, Winer J, Cachianes G, Li B, Leung DW 1991 The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol Endocrinol 5: 1806–1814.
Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC, Abraham JA 1991 The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem 266: 11947–11954.
Houck KA, Leung DW, Rowland AM, Winer J, Ferrara N 1992 Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J Biol Chem 267: 26031–26037.
Brock TA, Dvorak HF, Senger DR 1991 Tumor-secreted vascular permeability factor increases cytosolic Ca2+ and von Willebrand factor release in human endothelial cells. Am J Pathol 138: 213–221.
Monacci WT, Merrill MJ, Oldfield EH 1993 Expression of vascular permeability factor/vascular endothelial growth factor in normal rat tissues. Am J Physiol 264:C995–C1002.
Nagy JA, Brown LF, Senger DR, Lanir N, Van De Water L, Dvorak AM, Dvorak HF 1988 Pathogenesis of tumor stroma generation: a critical role for leaky blood vessels and fibrin deposition. Biochim Biophys Acta 948: 305–326.
Clauss M, Gerlach M, Gerlach H, Brett J, Wang F, Familletti PC, Pan YC, Olander JV, Connolly DT, Stern D 1990 Vascular permeability factor: a tumor-derived polypeptide that induces endothelial cell and monocyte procoagulant activity, and promotes monocyte migration. J Exp Med 172: 1535–1545.
Dvorak HF, Sioussat TM, Brown LF, Berse B, Nagy JA, Sotrel A, Manseau EJ, Van-de-Water L, Senger DR 1991 Distribution of vascular permeability factor (vascular endothelial growth factor) in tumors: concentration in tumor blood vessels. J Exp Med 174: 1275–1278.
Brown LF, Yeo KT, Berse B, Yeo TK, Senger DR, Dvorak HF, van-de-Water L 1992 Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J Exp Med 176: 1375–1379.
Brown LF, Berse B, Tognazzi K, Manseau EJ, Van-de-Water L, Senger DR, Dvorak HF, Rosen S 1992 Vascular permeability factor mRNA and protein expression in human kidney. Kidney Int 42: 1457–1461.
Hallman N, Norio R, Kouvalainen K 1967 Main features of the congenital nephrotic syndrome. Acta Paediatr Fenn 172( suppl ) 75–78.
Huttunen N-P 1976 Congenital nephrotic syndrome of Finnish type. A study of 75 patients. Arch Dis Child 51: 344–357.
Rapola J 1987 Congenital nephrotic syndrome. Pediatr Nephrol 1: 441–446.
Holmberg C, Antikainen M, Rönnholm K, Ala-Houhala M, Jalanko H 1995 Management of the congenital nephrotic syndrome of the Finnish type. Pediatr Nephrol 9: 87–93.
Striker GE, Striker LJ 1985 Glomerular cell culture. Lab Invest 53: 122–131.
Holthöfer H, Sainio K, Miettinen A 1991 Rat glomerular cells do not express podocytic markers when cultured in vitro. Lab Invest 65: 548–557.
Dekan G, Miettinen A, Schnabel E, Farquhar MG 1990 Binding of monoclonal antibodies to glomerular endothelium, slit membranes, and epithelium after in vivo injection. Am J Pathol 137: 913–927.
Holthöfer H, DeCandido S, Schlondorff D 1990 Identification of specific glomerular cell types in culture by use of lectin and antibody binding. Cell Differ Dev 30: 181–194.
Miettinen A, Dekan G, Farquhar MG 1990 Monoclonal antibodies against membrane proteins of rat glomerulus. Am J Pathol 137: 929–944.
Chirgwin JM, Przybyla AE, MacDonald RJ, Rutter WJ 1979 Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry 18: 5294–5299.
Sambrook J, Fritsch EF, Maniatis T 1989 Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY pp
Cunningham MW, Harris DW, Mundy CR 1990 In vitro labelling. In: Slater RJ (eds) Radioisotopes in Biology. Oxford University Press, Oxford, pp 137–191.
Wilcox JN 1993 Fundamental principles of in situ hybridization. J Histochem Cytochem 41: 1725–1733.
Berse B, Brown LF, Van-de-Water L, Dvorak HF, Senger DR 1992 Vascular permeability factor (vascular endothelial growth factor) gene is expressed differentially in normal tissues, macrophages, and tumors. Mol Biol Cell 3: 211–220.
Iijima K, Yoshikawa N, Connolly DT, Nakamura H 1993 Human mesangial cells and peripheral blood mononuclear cells produce vascular permeability factor. Kidney Int 44: 959–966.
Männikkö M, Kestilä M, Holmberg C, Norio R, Ryynänen M, Olsen A, Peltonen L, Tryggvason K 1995 Fine mapping and haplotype analysis of the locus for congenital nephrotic syndrome on chromosome 19q13.1. Am J Hum Genet 57: 1377–1383.
Mahan JD, Mauer SM, Sibley RK, Vernier RI 1984 Congenital nephrotic syndrome: evolution of medical management and results of renal transplantation. J Pediatr 105: 549–557.
Daniels BS 1993 The role of the glomerular epithelial cell in the maintenance of the glomerular filtration barrier. Am J Nephrol 13: 318–323.
Simon M, Grone HJ, Johren O, Kullmer J, Plate KH, Risau W, Fuchs E 1995 Expression of vascular endothelial growth factor and its receptors in human renal ontogenesis and in adult kidney. Am J Physiol 268:F240–F250.
Kaipainen A, Korhonen J, Pajusola K, Aprelikova O, Persico MG, Terman BI, Alitalo K 1993 The related FLT4, FLT1, and KDR receptor tyrosine kinases show distinct expression patterns in human fetal endothelial cells. J Exp Med 178: 2077–2088.
Breier G, Albrecht U, Sterrer S, Risau W 1992 Expression of vascular endothelial growth factor during embryonic angiogenesis and endothelial cell differentiation. Development 114: 521–532.
Acknowledgements
The authors thank Riitta Väisänen for skillful technical assistance.
Author information
Authors and Affiliations
Additional information
Supported by grants from the Finnish Cancer Foundation, Finnish Foundation for Pediatric Research and Päivikki and Sakari Sohlberg Foundation.
Rights and permissions
About this article
Cite this article
Haltia, A., Solin, ML., Jalanko, H. et al. Mechanisms of Proteinuria: Vascular Permeability Factor in Congenital Nephrotic Syndrome of the Finnish Type. Pediatr Res 40, 652–657 (1996). https://doi.org/10.1203/00006450-199611000-00002
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199611000-00002