Abstract
Previous studies within our research group have indicated that the hormonal influences on whole body energy expenditure may be modified in severely head-injured children. The aim of this study was to examine plasma concentrations of nonesterified fatty acids (NEFA) and the hormonal and metabolic mediators which influence these to determine whether there is a similarly modified effect on fat metabolism. A total of 64 serial measurements were made in 21 fasting severely head-injured children aged 2-15 y (Glasgow Coma Score ≤8) who were receiving neurointensive care. Circulating NEFA, ketone bodies, and lactate concentrations were analyzed using microenzymatic or electrochemical techniques. Plasma concentrations of adrenaline and insulin were measured using radioenzymatic and RIA techniques, respectively. Net fat oxidation rates were determined using indirect calorimetry. Plasma NEFA concentrations showed a significant positive relationship with both net fat oxidation rates (p = 0.02) and log ketone body concentrations(p = 0.008), indicating that NEFA concentrations were significantly related with utilization. When compared with reference values for normal resting adults, 59 (92%) adrenaline measurements were elevated, whereas only 8(12%) NEFA values lay above the reference range. Surprisingly, between children, there was a significant negative relationship between NEFA and adrenaline concentrations, even after allowing for the effects of insulin and lactate (p = 0.015). Both plasma NEFA and adrenaline concentrations were significantly related with Glasgo Coma Score (p = 0.04,p = 0.007, respectively), the most severely injured children having the lowest NEFA and highest adrenaline concentrations. The mechanisms underlying these metabolic changes may be related to the severity of head injury and may involve changes in triglyceride/NEFA cycling and/or peripheral effects on adrenergic receptors. If children are to be treated effectively after trauma, it is important to discover the mechanism of these changes which must reflect a fundamental alteration in metabolism.
Similar content being viewed by others
Main
Head injury is the most important cause of mortality and long-term morbidity in children over 1 y of age in the Western world, accounting for 25% of all deaths between the ages of 5 and 15 y(1). We have shown in a previous study that children, in general, are not hypermetabolic after severe head injury and that the whole body metabolic rate is positively associated with plasma concentrations of adrenaline. However, the stimulatory effect of adrenaline on whole body energy expenditure appears to be modified in critically head-injured children(2). To investigate the metabolic actions of adrenaline further, the effects of the hormone on lipolysis and utilization of NEFA were examined in the same group of head-injured children.
There is evidence in human adults to suggest that NEFA may be the preferred fuel for oxidation after severe trauma, and it is important, therefore, that NEFA are readily available to the body's tissues(3). Failure of such provision may result in increased protein catabolism leading to tissue wasting and an increased susceptibility to infection(4). Thus, the study of NEFA metabolism may have important implications for the nutritional management of head-injured children.
The net output of NEFA from adipose tissue represents a balance between the breakdown of triacylglycerols (lipolysis) and the esterification of NEFA, which occur simultaneously. The most important stimuli for hormone-sensitive lipase, which catalyzes lipolysis, are the catecholamines(5). Glucagon and ACTH have been found to stimulate lipolysis in vitro, but their effects are minimal in vivo(5). Insulin is probably the most important inhibitor of lipolysis(5). Glucose can also inhibit lipolysis independent of insulin action(6).
With the exception of erythrocytes, renal medulla, and brain, all tissues utilize NEFA as an important energy fuel. The uptake of NEFA from plasma is not under hormonal control, and under normal circumstances, the rate of utilization is directly proportional to the plasma concentration. Plasma NEFA concentrations provide a measure of the net rate of NEFA entry into the circulation together with a measure of their subsequent cellular uptake(7).
The aims of the current study were to make serial measurements of plasma NEFA concentrations to give an indication of net release of NEFA from adipose tissue and subsequent cell utilization. The relationships between NEFA concentrations and plasma adrenaline and insulin concentrations were investigated.
METHODS
Subjects. The study was performed in 21 fasting children who had sustained a severe head injury and were receiving neurointensive care. The mean age was 7.8 y with a range of 2 to 15 y. Eighteen children had isolated head injuries and three children had other associated injuries. Associated injuries were defined as injuries severe enough to warrant hospital admission in their own right which is approximately equivalent to an Injury Severity Score of ≥9(8). In all cases the head injury was the most serious injury sustained. The criterion for admission to the study was a GCS equal to or less than 8(9). In children aged less than 4 y, the adaptation of the GCS described by James and Trauner(10) was used. The median GCS was 6, range 3 to 8. Further clinical details of the children are given in Table 1. Ethical approval for the study was granted by the Joint Ethics Committee of Newcastle Health Authority and University of Newcastle upon Tyne, and informed written consent was obtained from the parents.
Management. The clinical care of the children remained the responsibility of the admitting neurosurgical and anesthetic teams. All children received elective intermittent positive pressure ventilation with mild hyperventilation, fractional inspired O2 concentration 0.3-0.35, with the arterial Pco2 being maintained between 3.5 and 4.5 kPa.
The children were sedated with continuous i.v. infusions of fentanyl (mean 3.0, range 0.9-8.4 μg kg-1 h-1); 15 children received a simultaneous infusion of midazolam (mean 105, range 34-229 μg kg-1 h-1). Doses of sedative drugs were varied within each child according to the clinical indications for sedation. Four children received an infusion of propofol (mean 4.3, range 1.6-8.6 mg kg-1 h-1) alone at some period during intensive care. All children received muscle relaxants, either pancuronium or vecuronium. Five children were given dopamine (mean 0.41, range 0.06-0.80 mg kg-1 h-1). No child received steroids.
Intravenous crystalloid fluids (0.9% saline or 0.18% saline/4% dextrose) were administered at maintenance requirements or with mild fluid restriction(75% of requirements). The mean glucose infusion rate was 1.4 mg kg-1 min-1 with a range of 0.4 to 2.3 mg kg-1 min-1. All children had a urinary drainage catheter and peripheral arterial catheter inserted.
Procedure. Serial measurements of arterial plasma NEFA concentrations together with circulating concentrations of adrenaline, insulin, β-hydroxybutyrate, acetoacetate and lactate were made in each child as soon as possible after admission to the intensive care unit. Measurements of gaseous exchange together with urinary nitrogen excretion were made using indirect calorimetry. Rates of net fat oxidation were calculated. Measurements were repeated every 6-24 h until the child was no longer receiving neurointensive care. All measurements were made during periods of clinical stability.
Measurements of metabolites were related to reference ranges for resting, fasting children(11), and measurements of hormones were related to reference ranges for resting, fasting adults(12), based on venous blood samples. There are some limitations in such comparisons.
Measurement of circulating intermediary metabolites and hormone concentrations. Serial 1-mL blood samples were taken from the indwelling arterial line. Aliquots of 750 μL of blood were placed in heparinized tubes for measurement of plasma NEFA, adrenaline, and insulin concentrations. Heparinized capillary tubes (40 μL) were filled with blood and deproteinized in tubes containing 200 μL of 5% perchloric acid for the measurement of ketone bodies. The samples were separated and the supernatant fluid was stored at -80°C until analysis. Aliquots of 150 μL of whole blood were placed in NaF tubes and separated, and the plasma lactate concentrations were analyzed immediately using a YSI 2300 Stat analyzer(Yellow Springs Instrument Co., Yellow Springs, OH), using an electrochemical method.
Plasma NEFA concentrations and whole blood β-hydroxybutyrate and acetoacetate concentrations were determined by microenzymatic methods using a Cobas fast centrifugal analyzer (Roche Products Ltd., Welwyn Garden City, UK)(13). A double-isotope radioenzymatic method was used for the assay of plasma adrenaline concentrations(14). Plasma insulin contrations were measured by RIA. The intraassay coefficients of variation were 1.5% NEFA, 8% adrenaline, 6% insulin, 4.1%β-hydroxybutyrate, 4% acetoacetate, and 7% for lactate.
Measurement of whole body net fat oxidation. Whole body net fat oxidation was measured using indirect calorimetry by a modified Douglas bag technique(15). This method has been described in detail in a previous paper(2). All children were ventilated with a Servo 900C ventilator using warmed humidified gases. A sample of the inspiratory and all the expiratory gases were collected into 5-L and 100-L metallized gas bags (Signal Instrument Co., Camberley, Surrey, UK), respectively, over an accurately timed period of 10-20 min, depending on the minute volume of the child. One liter of each of the expiratory and inspiratory gases was taken for analysis of O2 and CO2 concentrations.
Inspiratory and expiratory O2 concentrations were measured using a paramagnetic O2 analyzer (Servomex 540A, Servomex, Crowborough, Sussex, UK) and expiratory CO2 concentrations were measured using an infrared CO2 analyzer (Servomex PA404, Servomex). Gas samples were analyzed in triplicate to ascertain stable results.
The volume of the remaining expired gas was measured using a dry gas meter, DTM-200-4 (American Meter Company, distributed by International Gas Apparatus Ltd., Camberley, Surrey, UK), and expressed in terms of standard temperature and pressure, dry conditions. The complete indirect calorimetry system was evaluated using N2 and CO2 dilution techniques as described by Westenskow et al.(16). At an Fio2 of 0.3, the mean Vo2 recovery was 99.9% (SD ± 2%) and the mean Vco2 recovery was 99.4% (SD ± 2%). Twenty-four hour urine collections were made and urinary nitrogen content was analyzed using the Kjeldahl method(17) using an automated Kjeltac 1026 distillator (Perstorp Analytical Ltd, Basingstoke, Hampshire, UK).
Calculations. Whole body net fat oxidation was calculated from measurements of Vo2, Vco2, and urinary nitrogen excretion using the equations:
Statistical analysis. Circulating plasma adrenaline, insulin, ketone bodies, and lactate concentrations had skewed distributions and underwent logarithmic transformation before analysis. The data set is a mixture of cross-sectional and longitudinal data. To examine the between-child relationships between different variables, the data were analyzed using multilevel models(19) fitted using the ML3 program(20). This methodology is related to multiple regression and produces similar regression coefficients but allows each child to contribute different numbers of observations. Values of p ≤ 0.05 were considered to be statistically significant. For graphical presentation, the between-child relationships were displayed using the means of each variable for each child.
RESULTS
A total of 64 serial measurements of circulating NEFA, adrenaline, insulin,β-hydroxybutyrate, acetoacetate, and lactate concentrations were made in the 21 children. Indirect calorimetry was performed for 54 measurements in 18 children. The mean number of measurements per child was three, range one to nine. The median duration of each study was 32 h, range 9-122 h. The median time between injury and the first measurement in all 21 children was 14 h, range 7-43 h. Further details of the results are given inTable 1. Further details of the statistical analysis are given in Table 2.
Plasma NEFA concentrations and net fat oxidation rates after severe head injury. Figure 1 shows plasma NEFA concentrations over the first 120 h after the head injury. Plasma NEFA concentrations ranged between 0.13 and 1.94 mmol L-1 with mean 0.86 mmol L-1 and were within the reference range for resting fasting children for 54 (85%) measurements, elevated for 8 (12%) measurements from eight children, and below the reference range for 2 (3%) measurements from one child.
Mean whole body net fat oxidation rate was 1.58 mg kg-1 min-1(range -0.46 to 2.95). If plasma NEFA concentrations are related to cell uptake and subsequent utilization after severe head injury, one would anticipate finding a positive relationship between the plasma NEFA concentration and whole body net fat oxidation. Using multilevel modeling, a statistically significant positive relationship was found between these two variables [p = 0.02, regression coefficient (c) = 1.36] when compared between children, indicating that NEFA concentrations are related to utilization in this group of head-injured children.
Relationship between plasma NEFA concentrations and ketone body concentrations after severe head injury. Blood ketone body concentrations ranged between 0.05 and 3.35 mmol L-1, median 0.68 mmol L-1. Figure 2 shows the between-child relationship between plasma NEFA concentration and log10 blood ketone body concentration. If plasma NEFA concentrations are related to utilization, one would expect to find a positive relationship between the two variables. A statistically significant positive relationship was found, p = 0.008, c= 0.75, indicating again that plasma NEFA concentrations are related to utilization in this group of head-injured children.
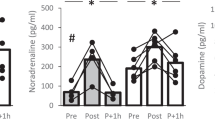
Hormonal mediators of lipolysis after severe head injury. The profiles of plasma adrenaline and insulin concentrations over the first 120 h after injury, together with reference ranges for normal resting fasting adults, are shown in Figure 3. In contrast to plasma NEFA concentrations, only three (5%) adrenaline measurements lay within the reference range, and 55 (92%) of measurements from all 21 children were elevated. Plasma insulin concentrations were highly variable, with 29 (46%) measurements lying within the reference range, seven (11%) measurements being elevated and 27 (43%) measurements being depressed.
The between-child relationship of plasma NEFA concentration and log adrenaline concentration is shown in Figure 4A. This examines how the mean level of adrenaline for each child relates to their mean level of NEFA. It was surprising to find a statistically significant negative relationship between the two variables, p = 0.006, c=-0.35.
Between-child relationship of plasma hormone concentrations and plasma NEFA concentrations. The graph shows the mean hormone concentrations for each child vs the mean plasma NEFA concentrations. (A) Adrenaline and (B) insulin are shown on a log scale. The children who received dopamine are shown by the symbol▴.
The between-child relationship of plasma NEFA concentration and log insulin concentration is shown in Figure 4B. As may be anticipated, there was a statistically significant negative relationship between the variables, p = 0.003, c = -0.40, consistent with the inhibitory effect of insulin on lipolysis.
The relationship between plasma NEFA and log adrenaline concentrations was investigated further by examining the effects of the severity of injury on these variables. Figure 5 shows the GCS on admission against mean plasma NEFA and adrenaline concentrations. Using multilevel modeling, it was found that plasma NEFA concentrations were positively related with GCS, p = 0.04, whereas log adrenaline concentrations were negatively related with GCS, p = 0.007. Thus, the most severely injured children, with the lowest GCS, had the lowest plasma NEFA concentrations and the highest adrenaline concentrations.
Plasma lactate levels are frequently elevated after severe trauma. Increased lactate concentrations give rise to increased cytosolic NADH/NAD+ ratios and increased production of α-glycerophosphate within adipose tissue, thus promoting reesterification. In the current study, the mean lactate concentration was 1.25 mmol L-1, range 0.3-5.0 mmol L-1. Using multilevel modeling, a significant negative relationship between log lactate and NEFA concentrations (p = 0.05, c =- 0.50) was found when comparison was made between children, consistent with the effect of lactate in promoting reesterification.
It was thought that the unexpected negative relationship observed between log adrenaline and NEFA may be accounted for by the effects of insulin and lactate. Therefore, plasma log insulin and log lactate concentrations were included as covariates in the multilevel model to allow for their effect on the relationship between NEFA and log adrenaline. However, it was found that the negative relationship between NEFA and log adrenaline persisted,p = 0.015, c = -0.33.
It should be noted that there was no effect of the child's age on any of the variables described. There was no evidence of an effect of varying doses of fentanyl or midazolam on net fat oxidation rates or on the relationship between log adrenaline and NEFA. Owing to the small numbers of children receiving dopamine and propofol, no analysis of the effect of these drugs was performed.
In summary, plasma NEFA concentrations were found to lie mostly within the reference range and to be significantly related with their utilization. Plasma adrenaline concentrations, however, were greatly elevated, and the degree of elevation was related to the severity of head injury. However, when comparisons were made between children, there was an unexpectednegative relationship between plasma NEFA and adrenaline concentrations, which persisted despite allowing for the opposing effects of insulin and lactate. This indicates that the well documented stimulatory effects of adrenaline on lipolysis may be reduced in proportion to the severity of the head injury.
DISCUSSION
The ability to mobilize endogenous fat is essential for energy balance because NEFA normally serve as a major energy source in the postabsorptive state. Despite elevated levels of plasma adrenaline after severe head injury, there was no evidence of increased availability of NEFA for subsequent oxidation and energy production in this group of head-injured children.
Studies of trauma in adult humans have shown that plasma concentrations of NEFA are increased or close to the normal range, although there is no close relationship with the severity of injury(21). In our study, NEFA levels lay mainly within the reference range for normal resting fasting children. There was a positive relationship between NEFA levels and GCS, the most severely injured children having the lowest NEFA concentrations. To the best of our knowledge, this relationship has not been described before.
Studies of trauma and isolated head injury in adults have shown plasma catecholamine levels to be positively related to the severity of injury(22, 23). The results of our study are in agreement with this. A total of 92% of plasma adrenaline levels were ≥0.5 nmol L-1. This is the threshold adrenaline concentration found to be associated with stimulation of lipolysis in a study of healthy adults infused with adrenaline at varying concentrations(6).
Under normal physiologic conditions, NEFA turnover may be evaluated by measuring plasma NEFA concentration. However, the situation is rather more complex, because Groop et al. (1991) have shown that, although plasma NEFA oxidation is primarily determined by plasma NEFA concentrations, net fat oxidation, which includes both oxidation of plasma NEFA and oxidation of intracellular lipids, is regulated by both plasma NEFA and insulin levels(24). Nordenstrom et al.(25) found that plasma NEFA concentrations were significantly correlated with net fat oxidation rates but not with fat turnover rates in their study of 18 traumatized and/or septic adult patients. Frayn et al.(26) showed that fat oxidation rates in injured patients were high in relation to plasma NEFA concentrations. In our study, however, the plasma NEFA concentration was positively related with both net fat oxidation and blood ketone body concentrations, indicating that plasma NEFA concentration was significantly related with utilization.
The net fat oxidation rate in a normal fasting adult man averages 1.37 mg kg-1 min-1(27). Unfortunately, there are no reference values available for normal children. In the children in our study, mean whole body net fat oxidation rate was 1.58 mg kg-1 min-1. In view of the increased energy expenditure per kg of body weight observed in children, it may be expected that net fat oxidation rates would be higher in normal children than in normal adults. It would appear, therefore, that the net fat oxidation rates observed in our group of head-injured children were unlikely to be significantly elevated.
The elevated plasma levels of adrenaline observed in these head-injured children would provide a strong lipolytic drive, and yet, plasma concentrations of NEFA were not elevated. Plasma NEFA concentrations indicate only net NEFA appearance in the plasma and do not provide information about reesterification occurring within adipose tissue. Unexpectedly low plasma NEFA concentrations may result either from increased rates of intracellular reesterification or a modified lipolytic response to catecholamines.
Intracellular recycling of triacylglycerols and NEFA. Intracellular recycling of triacylglycerols and NEFA is well described in normal adipose tissue. Lipolysis is stimulated by catecholamines, and reesterification is stimulated largely by insulin. Catecholamines may also increase reesterification themselves by increasing the intracellular NEFA concentration and by increasing glucose uptake by the adipocytes(5). Sympathetic stimulation of white adipose tissue leads to vasoconstriction which results in impairment of adipose tissue perfusion(28) and decreased availability of albumin for transport of NEFA released into the general circulation. This effect, which is accentuated by both local and general hypoxia (the latter operating through an increase in lactate), also results in favorable conditions for reesterification of NEFA within adipose tissue(29).
In this study, the significant negative relationship between NEFA and adrenaline concentrations occurring between children persisted despite allowing for the effects of circulating plasma insulin and lactate concentrations. This suggests that insulin and lactate concentrations do not account for the relatively low NEFA concentrations observed and the effect of catecholamines in stimulating reesterification themselves may be important.
There have been a number of studies of NEFA and glycerol turnover after trauma, and there has been considerable debate regarding alterations in the turnover and oxidation of fat. Several investigators have found increased rates of triglyceride/NEFA cycling after trauma or burns with little alteration in plasma NEFA concentrations(30, 31). Miyoshi et al.(5) showed that adrenaline acts directly to stimulate triglyceride/NEFA cycling and is not dependent on changes in insulin and/or glucose concentrations to stimulate reesterification. These observations are consistent with the findings in our study of head-injured children. However, Kurpad et al.(32) showed that noradrenaline infusions in healthy, fasting adult men resulted in a 3-4-fold increase in plasma NEFA concentrations with no evidence of increased triglyceride/NEFA cycling.
Attenuated lipolytic response to catecholamines. Relatively low plasma NEFA concentrations and decreased fat utilization may also arise from an attenuated response of lipolysis to catecholamines. Human adipocytes possess both stimulatory β-receptors and inhibitoryα2-receptors with the activity of the former normally predominating. The responsiveness of lipolysis to catecholamines is partly regulated by the degree of stimulation of α-relative toβ-adrenergic receptors(33). It is of interest to note that in infants and children, the inhibitory effects on lipolysis ofα2-adrenergic receptor stimulation is considerably greater than in the adult(34).
Desensitization of adrenergic receptors may result in a reduced response to catecholamines. Desensitization of β-receptors has been described in adipocyte lipolysis from adults after trauma. However, there was no evidence of desensitization of α-receptors implying less predominance of stimulatory β effects from catecholamines(35). The increased inhibitory effects of α receptors in infants and children may amplify this effect.
Although increased triglyceride/NEFA cycling or desensitization of β receptors may explain the unexpectedly low NEFA concentrations observed, the finding of a significant negative relationship between plasma adrenaline and NEFA concentrations has not yet been adequately explained. Both plasma adrenaline and NEFA concentrations were significantly related to GCS. Thus, the most severely injured children had the lowest GCS, the lowest NEFA, and highest adrenaline concentrations. Metabolic changes after trauma involve alterations in both the peripheral and central control of substrate utilization and metabolic rate. As the brain is involved in the pathogenesis of many metabolic changes, it might be predicted that damage to the brain would alter their pattern and/or their magnitude.
There have been few studies looking at fat metabolism after head injury. A study of severely head-injured adults showed that plasma concentrations of NEFA were significantly lower and plasma adrenaline levels were higher than in extracranially injured adults(36). A recent study by Petersen et al.(37) showed that fat mobilization and reesterification were decreased in adults with multiple trauma including the head compared with adults with multiple trauma not including the head. This implies a direct influence of head injury on the control of peripheral metabolism.
Whatever the mechanisms involved, the changes in fat metabolism observed in the present study after severe head injury seem to give rise to a reduction in the ability to mobilize fat stores. This may account for the increase in protein catabolism described after head injury in adults(38) and children(39) and has implications for the nutritional management of head-injured patients. It is clear that, if children are to be treated effectively after trauma, it is important to discover the mechanisms of these changes which must reflect a fundamental alteration in metabolism. NEFA and glycerol turnover studies together with indirect calorimetry and in vitro receptor studies may be useful in the further elucidation of this problem.

Abbreviations
- NEFA:
-
nonesterified fatty acids
- GCS:
-
Glasgow Coma Score
- Vo2:
-
O2 consumption in liters min-1
- Vco2:
-
CO2 production in liters min-1
- VE:
-
expired volume in liters min-1 at STPD
- STPD:
-
standard temperature and pressure, dry conditions
- Fio2:
-
proportion of O2 in inspiratory gas
- Feo2:
-
proportion of O2 in mixed expiratory gas
- Feco2:
-
proportion of CO2 in mixed expiratory gas
- N:
-
nitrogen excretion in g min-1
- F:
-
net fat oxidation in g min-1
References
Sharples PM, Storey A, Aynsley-Green A, Eyre JA 1990 Avoidable factors contributing to death of children with head injury. BMJ 300: 87–91.
Matthews DSF, Aynsley-Green A, Matthews JNS, Bullock RE, Cooper BG, Eyre JA 1995 The effect of severe head injury on whole body energy expenditure and its possible hormonal mediators in children. Pediatr Res 37: 409–417.
Little RA, Stoner HB, Frayn KN 1981 Substrate oxidation shortly after accidental injury in man. Clin Sci 61: 789–791.
Dionigi R, Gnes F, Bonera A, Dominioni L 1979 Nutrition and infection. J Parenter Enteral Nutr 3: 62–68.
Miyoshi H, Shulman GI, Peters EJ, Wolfe RR 1988 Hormonal control of substrate cycling in humans. J Clin Invest 81: 1545–1555.
Clutter WE, Bier DM, Shah SD, Cryer PE 1980 Epinephrine plasma metabolic clearance rates and physiologic thresholds for metabolic and hemodynamic actions in man. J Clin Invest 66: 94–101.
Armstrong DT, Steele R, Altszuler N, Dunn A, Bishop JS, De Bodo RC 1961 Regulation of plasma free fatty acid turnover. Am J Physiol 201: 9–15.
Baker SP, O'Neill B, Haddon W, Long W 1974 The Injury Severity Score: A method for describing patients with multiple injuries and evaluating emergency care. J Trauma 14: 187–196.
Teasdale G, Jennett B 1974 Assessment of coma and impaired consciousness. Lancet 2: 81–84.
James HE, Trauner DA 1985 The Glasgow Coma Scale. In: James HE, Anas NG, Perkin RM (eds) Brain Insults in Infants and Children. Grune & Stratton, London, pp 179–182.
Ranke MB 1992 Functional Endocrinologic Diagnostics in Children and Adolescents. J & J Verlag, Mannheim, 176
Young DS 1987 Implementation of SI Units for clinical laboratory data. Ann Intern Med 106: 114–129.
Harrison J, Hodson AW, Skillen AW, Stappenbeck R, Agius L, Alberti KGMM 1988 Blood glucose, lactate, pyruvate, glycerol, 3-hydroxybutyrate and acetoacetate measurements in man using a centrifugal analyser with a fluorimetric attachment. J Clin Chem Clin Biochem 26: 141–146.
Brown MJ, Jenner DA 1981 Novel double-isotope technique for enzymatic assay of catecholamines, permitting high precision, sensitivity and plasma sample capacity. Clin Sci 61: 591–598.
Douglas CG 1911 A method for determining the total respiratory exchange in man. Proc Physiol Soc 17-: 18
Westenskow DR, Cutler CA, Wallace WD 1984 Instrumentation for monitoring gas exchange and metabolic rate in critically ill patients. Crit Care Med 12: 183–187.
Fleck A, Munro HN 1965 The determination of organic nitrogen in biological materials. A review. Clin Chim Acta 11: 2–12.
Frayn KN 1983 Calculation of substrate oxidation ratesin vivo from gaseous exchange. J Appl Physiol 55: 628–634.
Goldstein H 1995 Multilevel Statistical Models. Edward Arnold, London, 87–94.
Prosser R, Rasbash J, Goldstein H 1991 ML3: Software for Three-Level Analysis. Institute of Education, London, 73–78.
Stoner HB, Frayn KN, Barton RN, Threlfall CJ, Little RA 1979 The relationships between plasma substrates and hormones and the severity of injury in 277 recently injured patients. Clin Sci 56: 563–573.
Davies CL, Newman RJ, Molyneux SG, Grahame-Smith DG 1984 The relationship between plasma catecholamines and severity of injury in man. J Trauma 24: 99–105.
Clifton GL, Ziegler MG, Grossman RG 1981 Circulating catecholamines and sympathetic activity after head injury. Neurosurgery 8: 10–14.
Groop LC, Bonadonna RC, Shank M, Petrides AS, DeFronzo RA 1991 Role of free fatty acids and insulin in determining free fatty acid and lipid oxidation in man. J Clin Invest 87: 83–89.
Nordenstrom J, Carpentier Y, Askanazi J, Robin AP, Elwyn DH, Hensle TW, Kinney JM 1983 Free fatty acid mobilization and oxidation during total parenteral nutrition in trauma and infection. Ann Surg 198: 725–735.
Frayn KN, Little RA, Stoner HB, Galasko CSB 1984 Metabolic control in non-septic patients with musculoskeletal injuries. Injury 16: 73–79.
Elia M, Zed C, Neale G, Livesey G 1987 The energy cost of triglyceride-fatty acid recycling in nonobese subjects after an overnight fast and four days of starvation. Metabolism 36: 251–255.
Stoner HB, Matthews J 1967 Studies on the mechanism of shock. Fat mobilization after injury. Br J Exp Pathol 48: 58–65.
Fredholm BB 1971 The effect of lactate in canine subcutaneous adipose tissue in situ. Acta Physiol Scand 81: 110–123.
Jeevanandam M, Young DH, Schiller WR 1990 Nutritional impact on the energy cost of fat fuel mobilization in polytrauma victims. J Trauma 30: 147–154.
Wolfe RR, Herndon DN, Jahoor F, Miyoshi H, Wolfe M 1987 Effect of severe burn injury on substrate cycling by glucose and fatty acids. N Engl J Med 317: 403–408.
Kurpad A, Khan K, Calder AG, Coppack S, Frayn K, Macdonald I, Elia M 1994 Effect of noradrenaline on glycerol turnover and lipolysis whole body and subcutaneous adipose tissue in humansin vivo. Clin Sci 86: 177–184.
Burns TW, Langley PE 1970 Lipolysis by human adipose tissue: the role of cyclic 3,5-adenosine monophosphate and adrenergic receptor sites. J Lab Clin Med 75: 983–997.
Rosenbaum M, Presta E, Hirsch J, Leibel RL 1991 Regional differences in adreno-receptor status of adipose tissue in adults and prepubertal children. J Clin Endocrinol Metab 73: 341–347.
Forse RA, Leibel R, Askanazi J, Hirsch J, Kinney JM 1987 Adrenergic control of adipocyte lipolysis in trauma and sepsis. Ann Surg 6: 744–751.
Hadfield JM, Little RA, Jones RAC 1992 Measured energy expenditure and plasma substrate and hormonal changes after severe head injury. Injury 23: 177–182.
Petersen SR, Jeevanandam M, Harrington T 1993 Is the metabolic response to injury different with or without severe head injury? Significance of plasma glutamine levels. J Trauma 34: 653–661.
Hadfield JM, Little RA 1992 Substrate oxidation and the contribution of protein oxidation to energy expenditure after severe head injury. Injury 23: 183–186.
Andrassy RJ, Dubois T 1985 Modified Injury Severity Scale and concurrent steroid therapy: independent correlates of negative nitrogen balance in pediatric trauma. J Pediatr Surg 20: 799–802.
Acknowledgements
The authors thank the medical and nursing staff of the intensive care unit, and the consultant neurosurgeons who allowed them to study their patients. We acknowledge the assistance of the following: Dr. J. N. S. Matthews, Department of Medical Statistics, Newcastle University, for statistical advice, Professor K. Bartlett, Department of Child Health, Newcastle University, for helpful comments about the manuscript, A. McGann, Department of Child Health, Newcastle University, with metabolite assays, L. Ashworth and Professor K. G. M. M. Alberti, Diabetic Research Group, Newcastle University, with insulin assays, and M. Ashby and Professor M. Brown, Department of Clinical Pharmacology, Addenbrooke's Hospital, Cambridge, with adrenaline assays.
Author information
Authors and Affiliations
Additional information
Supported in part by the Scientific and Research Committee of Newcastle Health Authority and by the Medical Research Council (D.S.F.M.) with additional financial support from The Intensive Care Society, The Buttle Trust, The Mason Medical Foundation, The Peel Medical Research Trust, and CHILD.
Rights and permissions
About this article
Cite this article
Matthews, D., Aynsley-Green, A. & Eyre, J. Modified Hormonal Effects on Fat Metabolism after Severe Head Injury in Children. Pediatr Res 39, 1012–1019 (1996). https://doi.org/10.1203/00006450-199606000-00014
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1203/00006450-199606000-00014
This article is cited by
-
Voeding bij ernstig zieke kinderen
Tijdschrift voor kindergeneeskunde (2001)