Abstract
The Diels–Alder reaction has been widely used in synthetic organic chemistry since its discovery in 1928. The catalyst-free nature, functional group tolerance and high efficiency of the Diels–Alder reaction also make it promising for the fabrication of functional polymeric materials. In particular, a large variety of functional polyphenylenes (polymer structures mainly consisting of phenylenes) and ladder polymers (double-stranded polymers with periodic linkages connecting the strands) have been obtained by this method, offering potential applications such as polymer electrolyte membranes and gas separation. More recently, tailor-made polyphenylenes prepared by Diels–Alder polymerization have been used as precursors of structurally well-defined graphene nanoribbons (ribbon-shaped nanometer-wide graphene segments) with different widths, exhibiting long lengths (>600 nm) and tunable electronic bandgaps. This article provides a comprehensive review of the use of Diels–Alder polymerization to build functional polyphenylenes, ladder polymers and graphene nanoribbons.
Similar content being viewed by others
Introduction
The Diels–Alder (D–A) reaction, named after Otto Diels and Kurt Alder,1 is clearly one of the most important and commonly used organic processes in synthetic organic chemistry.2 This concerted [4+2] cycloaddition leads to six-membered-ring products with controllable stereochemistry. The D–A reaction is, in principle, metal/catalyst free, and it further features thermal reversibility, rapid kinetics, high versatility and wide functional group tolerance.3, 4 These characteristics make the D–A reaction a suitable choice for constructing a wide range of polymeric materials, including discrete and soft supramolecular networks, semirigid polyphenylenes (PPs), rigid ladder polymers and biocompatible and stimulus-responsive ‘smart’ materials, such as self-repairing and shape-memory gels.4, 5, 6, 7
PPs are a class of polymers mainly consisting of phenylene units, as represented by poly(para-phenylene) (PPP 1; Scheme 1), which are often substituted with solubilizing groups such as bulky alkyl chains and/or additional aryl groups. In the 1960s, Kovacic and Kyriakis8 investigated the Lewis acid-mediated oxidative aryl–aryl coupling of benzenes to form linear oligo(para-phenylene)s and PPP 1, albeit with structural defects, limited solubility and a low degree of polymerization (DP). Since then, there have been breakthroughs in the synthesis of various PPs via, for example, metal-catalyzed aryl–aryl couplings and the cyclotrimerization of alkynes, the thermal ring opening of biphenylenes and D–A reactions (vide infra).9, 10, 11 The topologies of PPs range from the above-mentioned one-dimensional polymers such as PPP 1 as molecular ‘wires’ to two-dimensional porous PPs12 and three-dimensional PP dendrimers.11 These PP materials are used in various applications including organic electronics, sensors, bioimaging and drug delivery.9, 10, 11, 13, 14, 15 From an electronic perspective, the benzene rings in PPP 1 are twisted away from each other due to the steric repulsion between the protons, which compromises the conjugation along the polymer chain. In 1991, Scherf and Müllen et al.16 succeeded in ‘locking’ the conformation of PPP 1 by covalently bridging neighboring phenylene rings, leading to the ladder-type poly(para-phenylene) 2.
Ladder polymers can be defined as double-stranded polymers with periodic linkages connecting the two strands, which resemble the rails and rungs of a ladder,17 for example, ladder-type poly(para-phenylene) 2 and the angular polyacene ladder polymer 3 (Scheme 1). Ladder polymers can be conventionally constructed through (1) one-pot polymerization, by which the two strands of ladder polymers are built up simultaneously, for instance, by repetitive D–A reactions and (2) a two-step sequence of polymerization and postannulation.18 In particular, fully conjugated ladder polymers, including ladder-type PPPs 2 and 3, are demonstrated to be promising semiconductor materials with outstanding stability, large coherent π-conjugation length,19 fast intrachain charge carrier mobility20 and long exciton diffusion length.21 The remarkable electronic properties of such ladder polymers make them suitable for applications in the field of organic (opto)electronics such as organic light-emitting diodes, organic field-effect transistors (FETs) and solid-state lasers.18, 22, 23, 24, 25
On the other hand, such fully conjugated ladder polymers can be regarded as the narrowest examples of graphene nanoribbons (GNRs), quasi-one-dimensional graphene segments with widths <100 nm and aspect ratios >10.26, 27, 28 Graphene is a two-dimensional allotrope of carbon exhibiting excellent electronic properties such as extremely high charge carrier mobility.29 Graphene is thus considered one of the most promising materials for future nanoelectronics. However, the lack of an electronic bandgap hinders the application of graphene as a semiconductor material, for example, in FETs. In contrast to the gapless graphene, GNRs, such as armchair GNR 4 (Scheme 1), have tunable bandgaps that depend mainly on their width and edge structures and are emerging as next-generation semiconductor materials.30, 31 Together with their high intrinsic charge carrier mobility, GNRs are thus attracting attention for applications in photovoltaic cells, optical sensors and logic gates.32, 33, 34, 35, 36 In recent years, tremendous progress has been made in the fabrication of GNRs through both top-down and bottom-up approaches. The top-down approach is usually realized by lithographic slicing of the graphene sheets and the unzipping of carbon nanotubes.37, 38, 39, 40, 41 However, this method provides GNRs with low yield and without structural control. In contrast, the bottom-up protocol, which is implemented via the solution-mediated or surface-assisted planarization of tailor-made PP precursors by intramolecular cyclodehydrogenation, offers structurally well-defined GNRs.26, 27
In this review, we shall describe the role of D–A polymerization in the synthesis of functional PPs (in the next section), ladder-type polymers (in the penultimate section) and GNRs (in the last section), including the latest updates in the fields. The readers are advised to consult previous reviews in the literature for more comprehensive information about PPs,9, 10, 11, 12, 13, 14, 15 ladder-type polymers18, 22, 23, 24, 25 and GNRs,26, 27, 28, 30, 31, 34 especially their preparation through other polymerization methods. Here, we highlight the key role of D–A polymerization in the synthesis of these three classes of polymer materials, which are closely related to each other but have seldom been discussed as a group.
Functional PPs via D–A polymerization
The A2B2-type D–A polymerization
The D–A reaction is an obvious protocol for a polymerization reaction to synthesize aromatic polymers, as it generates a six-membered ring product, which can then be aromatized. However, with its reversible nature, namely, the possible retro-D–A reaction of the intermediate products, it is possible to reach only D–A polymers with a relatively low degree of polymerization (DP), because of the thermodynamic limitations of the reactions. Polymers based on the D–A reactions of furan and maleimide generally possess DPs lower than 20.42 Nevertheless, it is possible to overcome this obstacle and achieve higher DPs by (1) enhancing the enthalpic driving force for the D–A cycloaddition, typically by simultaneous aromatization, for example, the D–A reaction of o-quinodimethane and N-phenylmaleimide43 (Scheme 2a), and/or (2) preprogramming an irreversible consecutive reaction such as the removal of a volatile small molecule or aromatization of the D–A adduct.44 A representative example is the reaction of 2,3,4,5-tetraphenyl-cyclopentadienone as a diene and diphenylacetylene as a dienophile, leading to the formation of an aromatic benzene ring after the removal of carbon monoxide to afford hexaphenylbenzene (Scheme 2b, see Table 1 for more specific examples).9, 45, 46
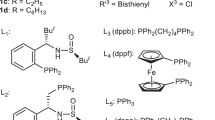
In 1966, Stille et al.47 developed an A2B2-type D–A polymerization using cyclopentadienone (Cp) units as the diene to synthesize phenylated PPs 5 and 6, where the polymer backbones consisted of random mixtures of para- and meta-phenylenes (Table 1). The synthesis was accomplished by the repetitive D–A reaction of bis-Cps as an A2 monomer and bisacetylene as a B2 monomer in toluene at 300 °C in a sealed tube, with a possible isomerization upon each cycloaddition step due to the asymmetrical diene structure of the bis-Cps. In this first attempt, bis-Cps linked with diphenyl (thio)ether or α,ω-diphenyl alkanes were reacted with m- and p-diethynylbenzenes (PP 5, with x=O or S and PP 6, with n=3 or 4 for L1; Table 1). In the following few years, the scope of the bis-Cp A2 monomers was extended, but no further progress in using other kinds of bisacetylene was made (PPs 5–7; Table 1).47, 48, 49, 50, 51, 52, 53, 54 Table 1 summarizes the different structures of L1 and L2, the estimated number of monomeric units in the D–A polymer (i.e., DP) and the thermal decomposition temperature (Td) of the representative PPs 5–14 reported in the literature.
Despite the successful demonstration of this A2B2-type synthesis of PPs by repetitive D–A reactions, there was no follow-up study on this subject for ~30 years, probably because of the lack of efficient synthetic protocols for bisacetylenes.55 After the booming development of the chemistry of metal-catalyzed cross-coupling reactions in the 1970s, in particular the Sonogashira–Hagihara coupling, Kumar and Neenan55 revisited and extended the scope of such D–A polymerizations in 1995. Bisacetylenes with different L2 linkers, such as trimethylsilyl and perfluorobenzene and benzophenone and thiophene, were successfully incorporated into this polymerization protocol under the milder reaction conditions of heating in cyclohexylbenzene at 200 °C (PPs 8–11; Table 1).
The highest DP of 137 was obtained when diphenyl ether and tetrafluoro-m-phenylene were used as linkers L1 and L2, respectively (PP 5; Table 1). Chromophores such as fluorene, triphenyl amine and quinoxaline could be directly incorporated into the polymer backbone, providing blue-emitting materials (PP 12; Table 1).56 Moreover, these materials demonstrated very high thermal stability with decomposition temperature (Td) values up to 550 °C (Table 1). The solubility of these phenylated PPs was surprisingly good in standard organic solvents such as tetrahydrofuran (THF), chloroform and dimethylformamide, which could be the result of random meta and para linkages in the polymer backbone established during the polymerization (Table 1). The high DP, stability and solubility rendered these materials attractive for applications as polymer membranes, which require high physical and chemical robustness for long-term use under harsh conditions.57
Polymer membrane applications of PPs from D–A polymerization
Gas separating membrane
PPs synthesized via A2B2-type D–A polymerization can be cast as films from common organic solvents.47, 55 The amorphous nature and large fractional free volume of the pure hydrocarbon PPs 7 and 13 were revealed by their broad peaks in X-ray scattering patterns, their density and their calculated van der Waals volume.58 These properties are important requirements for highly permeable membrane applications.59 Very recently, Cornelius and co-workers58 studied the gas permeability, solubility, diffusivity and selectivity of He, H2, O2, CO2, N2 and CH4 gases in membranes composed of PPs 7 and 13 as well as their copolymers (Table 1). These membranes displayed cutting-edge gas permeability/selectivity trade-offs. Namely, they possessed higher CO2 gas permeability (measured as pressure) than those of conventional devices built from PP oxide and polyimide, while still exhibiting comparable selectivity for CO2 over N2 (20 times greater permeability).
Polymer electrolyte membranes
Polymer electrolyte membranes (PEMs) are showing great potential as components of fuel cells and solar cells, as well as devices for electrolysis, dialysis and water splitting.60, 61 The standard material used in most cases is Nafion, a poly(tetrafluoroethylene) with pendant perfluorosulfonic acids that possesses low water uptake and high proton conductivity, but suffers from limited operation temperatures (0–80 °C), high cost and high fuel crossover. Hydrocarbon-based polymer electrolytes, because of their synthetic versatility, high chemical stability and relatively low cost, are emerging as an important class of alternative materials for PEMs in electrochemical applications.62 Since the first approach conducted by Cornelius, Loy and co-workers in 2005,63 a series of hydrocarbon-based PP polyelectrolytes, 15–17, have been synthesized and investigated.64, 65, 66, 67 These polyelectrolytes were obtained by the sulfonation63, 64, 66, 67 or bromination/amination65 of phenylated PPs 7, 13 and 14, which were subjected to functionalization after the initial D–A polymerization (Table 1 and Scheme 3).
The ion exchange capacity (IEC) of polyelectrolyte 15 varied between 0.98 and 2.2 mequiv g−1, corresponding to 0.8–2.1 sulfonic acid groups per repeating unit. These materials were soluble in highly polar aprotic solvents such as dimethylacetamide and N-methyl-2-pyrrolidone, but insoluble in nonpolar organic solvents and water.63 They were highly thermally stable with only 5% weight loss at 363–422 °C, and the glass transition temperature (Tg) was not observed before the decomposition temperature. In both the dry and wet forms of the membrane of 15, the Young’s modulus was ~6 times higher than that of a commercially available Nafion 117 membrane.63 Although the PEM of 15 revealed poorer proton conductivity than that of Nafion 117, its methanol and glucose permeability values were much smaller.64 With these intriguing properties, such PEMs were successfully examined in various applications, including hydrogen/methanol fuel cells,66 vanadium redox flow batteries68 and electrodialysis desalination.67 In particular, the electronic desalination of 1.0 L of 0.1 wt% NaCl using the PEM of 15 was completed within 44 min using 5.8 J g−1, which was much more efficient than the performance of the commercially available cation or anion exchange membranes PC-SK and PC-SA. The latter required 8.4 J g−1 and 79 min for the same volume and concentration of NaCl solution.67
Cornelius and co-workers65 also synthesized a cationic polyelectrolyte, 16, with the IEC of 0.93–1.57 mequiv g−1 through the postbromination/amination of methylated PP 13 (Table 1 and Scheme 3). Interestingly, the water uptake of the cationic polyelectrolyte 16 was roughly two times higher than that of its anionic analog 15 with similar IEC. Notably for an anion exchange membrane, the PEM of 16 exhibited a high hydroxide conductivity of up to 50 mS cm−1.
In 2014, Kim and co-workers69 described the synthesis of the polyelectrolyte 17, with an IEC of 1.49–2.34 mequiv g−1, by the postsulfonation of D–A polymerized poly(pentaphenylene sulfone) 14 (Table 1 and Scheme 3). The thermal stability of the anionic poly(pentaphenylene sulfone) polyelectrolyte 17 was slightly lower than that of the PP polyelectrolyte 15, with 5% weight loss at ~270–350 °C and with superior mechanical properties to those of Nafion 211. Interestingly, the PEM of 17 showed lower water uptake than that of 15, probably a result of selective sulfonation on the pendant phenyl rings due to the lowered reactivity of the phenylene backbone in the presence of electron-withdrawing sulfone groups. The PEM of 17 with an IEC of 2.34 mequiv g−1 provided higher proton conductivity than that of Nafion 212, leading to a slightly more efficient hydrogen fuel cell.
Holdcroft and co-workers70, 71 reported the synthesis of polyelectrolytes 19a–c by the D–A polymerization of the pre-sulfonated 4,4'-(1,4-phenylene)bis-(2,3,5-triphenylcyclo-penta-2,4-dien-1-one) 18 with 1,4-diethynylbenzene, 4,4'-diethynylbiphenyl and 1,4-diethynylnaphthalene, respectively (Scheme 4). This approach achieved precise numbers of functionalizations at specific positions, in contrast to PPs 15–17, which were prepared through rather random postsulfonation.70, 71 With very high DPs of up to 125 and polydispersion indices (PDIs) of 1.44–2.33, polymers 15a–c displayed good mechanical strength and thermal stability similar to those of their analogs 15 and 17. The IECs of polyelectrolyte 19a–c were as high as 3.47, 3.19 and 3.28 mequiv g−1, respectively.
With the larger hydrophobic bridges, the water uptake values of biphenylene- and naphthylene-spaced 19b and 19c were smaller than that of phenylene-spaced 19a, although still much larger (~6 and 9 times as high, respectively) than that of Nafion 211. Nevertheless, the remarkably high proton conductivity of the PEMs of 19b and 19c (172 and 268 mS cm−1, respectively, compared with 113 mS cm−1 for the PEM of Nafion 211 at 95% relative humidity, 80 °C) furnished remarkable performance of their hydrogen fuel cells. The hydrogen fuel cells of 19b and 19c exhibited 56% and 17%, respectively, greater peak power density than that of Nafion 211. Moreover, the durability of the hydrogen fuel cell using biphenylene-spaced 19b as the membrane material was more than four times greater than that of Nafion 211.
The hydrocarbon-based D–A phenylated PP PEMs with their superior chemical and thermal stability as well as mechanical strength could reach comparable and even higher performances compared with those of the most commonly used Nafion-based PEMs. Clearly, they are thus possible candidates for future PEM applications.
Hyperbranched PP by D–A polymerization
In contrast to the time-consuming step-by-step synthesis of a dendrimer from ABn building blocks,11, 14, 15 hyperbranched polymers could be constructed in a one-pot ‘uncontrolled’ polymerization of ABn-type monomers, in principle the same but unprotected, ideally forming a branch on every repeating unit. Molecularly defined dendrimers as well as dendritic and hyperbranched polymers establish a class of attractive materials in view of their unique properties derived from their branched three-dimensional architectures, such as the ability to accommodate a large number of functional groups, high solubility and low viscosity.72 PPs synthesized by repetitive D–A reactions can furnish highly substituted benzene building blocks whose molecular weights are higher than those achieved by other methods such as cross-coupling reactions.9 This ability makes the D–A reaction a very promising choice for the synthesis of hyperbranched phenylated PPs.
In parallel with the development of PP dendrimers from AB2-type 3,4-bis(4-ethynylphenyl)-2,5-diphenylcyclopentadienone-based building blocks 20, we have also worked on the direct D–A polymerization of monomers 20a–c to form hyperbranched phenylated PPs with DPs of ~45 and PDIs of 1.7–6.9 (Scheme 5).73 The resulting polymers exhibited high thermal stability with Td values higher than 550 °C and good solubility in toluene and benzene even without solubilizing substituents. To test the possibility of forming free-standing tubular structures of such hyperbranched polymeric materials, monomer 20d, with long alkyl chains, was D–A polymerized in a vertically nanochanneled aluminum membrane template at a temperature above 200 °C (Scheme 5).74 After removal of the template, the resulting material formed highly flexible and orderly aligned hollow nanotubes. They possessed a wall thickness ranging from 5 to 50 nm, an average diameter of 200 nm and a length up to 60 μm, corresponding to the thickness of the aluminum membrane used as the template (Figure 1a). Moreover, it was possible to form a highly porous carbon nanotube through direct carbonization of the hyperbranched polymer in the aluminum membrane by heating to 600 °C followed by removal of the template (Figure 1b). The pore size was large, up to 20 nm, which probably stemmed from cleavage of the alkyl chains of 20d during the carbonization.
Scanning electron microscope (SEM) images of (a) hyperbranched polyphenylene (PP) nanotubes of 20d and (b) the results after pyrolysis at 600 °C. Reprinted with permission from Zhi et al.74 Copyright 2005 John Wiley & Sons.
Voit and co-workers75 revisited this field and developed two more hyperbranched PP systems in 2006, namely, (1) extending the above-mentioned AB2 system from 20 through an AB+AB2-type D–A polymerization of 20c (AB2) and 21 (AB), and (2) an A2B3-type D–A polymerization of 4,4'-(1,4-phenylene)bis(2,3,5-triphenylcyclopenta-2,4-dien-1-one) (22) (A2) and 1,3,5-tris(phenylethynyl)benzene (23) (B3), related to the A2B2 systems described in sections 'The A2B2-type D–A polymerization' and 'Polymer membrane applications of PPs from D–A polymerization' (Scheme 5).75 A Mw as high as 74 kg mol−1 was achieved for the AB+AB2 system when the reaction was carried out at a ratio of 3:1 for 20c (AB2) and 21 (AB). The more AB linear segments 21 were used, the lower the resulting molecular weight became. On the other hand, the molecular weight of the A2B3 polymers was lower than that of the previous AB2 polymers. The nuclear magnetic resonance spectral analysis suggested that in this A2B3 system, the formation of the linear polymer was favored. The third B unit in B3 monomer 23 would react only when using a large excess of A2 monomer 22, thus providing a way to control the degree of branching of the resulting polymers.
Along the same lines, Shifrina and co-workers76 described hyperbranched pyridylphenylene polymers based on the A2B6-type D–A polymerization of 22 (A2) and 24 (B6) (Scheme 5). Gelation was observed when the reactions were carried out at a 3:1 ratio of A2 (22) and B6 (24) monomers, probably because severe crosslinking occurred at this exact stoichiometric ratio of A and B units. The highest detectable Mw of 80 kg mol−1 was achieved when using a 1:1 ratio of A2 (22) and B6 (24) building blocks. Based on the nuclear magnetic resonance spectra, the degree of branching of this A2B6 system could be controlled. By adjustment of the stoichiometry of the A and B units, a dominant three- to fivefold reaction of the B6 building blocks could be achieved.
These hyperbranched PPs obtained by D–A polymerization featured high-molecular weights and controllable degrees of branching and offered the possibility of introducing high numbers of functional groups. This new class of functionalized PPs would be intriguing for future applications, for example, in the PEM materials mentioned in section 'Polymer membrane applications of PPs from D–A polymerization'.
Ladder polymers synthesized by D–A reactions
The D–A reactions have a historical importance for the development of ladder polymers. With the concerted mechanism of D–A reactions, the two strands of ladder polymer could be built up simultaneously. This simultaneous construction efficiently reduces the possibility of side reactions, which is a great advantage for the synthesis of defect-free ladder polymers. As early as 1962, Bailey et al.77 reported the synthesis of ladder polymer 28 from one of the simplest bisdienes, 2-vinylbutadiene (25), by D–A polymerization (Scheme 6).
The D–A reaction between bisdiene 25 and p-benzoquinone (26) at room temperature probably formed the 1:1 adduct 27, which has both diene and dienophile moieties. Then, 27 further homopolymerized as an AB-type monomer in a refluxing CCl4 solution to afford ladder polymer 28. Unfortunately, the resulting structure 28 could barely be dissolved in hot chlorinated organic solvents, and the soluble part was a mixture mainly containing dimers and trimers.
Since this example, several successful D–A polymerization systems have been applied for the synthesis of ladder polymers possessing rigid and semirigid structures. In the following sections, these products will be categorized by the different bisdienes used in their synthesis.
Monomers containing 1,2,5,6-tetramethylenecyclooctane and 1,2,4,5-tetramethylenecyclohexane structures, toward the synthesis of rigid and semiflexible ladder polymers
Tetramethylenecycloalkanes with rigid and bent structures, such as that of 29, or more flexible cyclooctadiene structures, such as those of 30 and 31, can react as bisdienes and form ladder polymers with unprecedented architectures (Scheme 7). In 1987, Williams, Stoddart and co-workers78 synthesized a cyclic ‘molecular belt’ from two 2,3,5,6-tetramethylene-7-oxabicyclo[2.2.1]heptanes (29a) and two 1,4,5,8-tetrahydro-1,4:5,8-diepoxyanthracenes (32) by A2B2 type D–A reaction under high-pressure conditions, which were made possible by the rigid bent structures of 29a and 32, as well as the high stereoselectivity of the D–A reaction. We used a similar bisdiene 29b and bisalkene 33 to construct ladder polymer 34.79, 80 In our approach, a 1:1 mixture of the syn-isomer of bisalkene 33 and its anti-isomer that would form a more extended D–A adduct were used to suppress the possible formation of small cyclic products. The A2B2 type D–A polymerization of 29b and 33 was achieved in dichloromethane at 65 °C under a high pressure of 7.5 kbar. The obtained polyacene precursor 34 was soluble in common organic solvents such as THF and acetone with DP up to 17 and PDI of 2.8–3.2. The thermal stability of this polymer was low, with a Td of ~200 °C.
In addition, we examined monomers 30 and 31, which feature 1,2,5,6-tetramethylenecyclooctane structures, and found that the reactivity of 31 toward D–A polymerization was higher than that of 30 (Scheme 7).81 The D–A adducts of monomers 30 and 31 contain a semiflexible cyclooctadiene moiety as a ‘molecular hinge’ that can adopt chair, boat and twist-boat conformations. This hinge moiety gave the D–A ladder polymers built from bisdiene 30 or 31 a higher degree of conformational freedom than those built from the rigid bisdiene 29. The A2B2-type D–A polymerization of bisdiene 31 and alkyne 35 (reacted as an equivalent of bisalkene) under high pressure (8 kbar) resulted in a complicated dynamic equilibrium, in which the linear ladder polymer 36 could be isolated in 80% yield at 100 °C with the highest DP of 27 (Scheme 7).82 On the other hand, at 50 °C, the 1+1 cyclic cage product 37 together with other larger cyclic compounds were favored, with up to 60% isolated yield.
Monomers containing cyclopentadienone moieties, toward the synthesis of fully conjugated ladder-type polyfluoranthene
The use of Cp as the diene for the synthesis of fully conjugated ladder polymers was first approached by Stille et al. in 1970.83 In this approach, the AB-type Cp-based monomer 38 with phenyl side groups was D–A polymerized and in situ aromatized by air to afford the ladder-type polyfluoranthene 39, featuring a similar structure to that of the belt region of C60 (Scheme 8). Ladder polymer 39, which has a fully conjugated aromatic structure, could also be considered the first synthesized GNR with defined defects. Polyfluoranthene 39 was thermally stable with 10% weight loss at ~400 °C. However, it displayed very low solubility and could be only partially dissolved in benzene. Schlüter and Löffler et al.84 approached the solubility and characterization problem of ladder polymer 39 in 1994 by replacing the phenyl side groups with bridged flexible alkyl chains as well as isolating the non-aromatized polymer precursor 41. The addition of an antioxidant during the D–A polymerization of AB-type monomer 40 successfully hindered the otherwise simultaneous aromatization and allowed the isolation of ladder polymer 41. The nonplanar structure and bridged alkyl chains of 41 rendered it well soluble in THF and chloroform. Polymer 41 was thus characterized by 1H and 13C nuclear magnetic resonance, ultraviolet–visible (UV–vis) spectroscopy and gel permission chromatography (GPC) to reveal its DP and PDI of 7–14 and 2.5–2.8, respectively. The planarization of the polymer precursor 41 toward the fully conjugated fluoranthene ladder polymer 42 was achieved by oxidation in the presence of DDQ (2,3-dichloro-5,6-dicyano-p-benzoquinone), demonstrating an optical absorption of oligomers extending up to ca. 600 nm. Nevertheless, the high-molecular-weight fraction of 42 still gave low solubility in common organic solvents such as THF and chloroform.
In situ generation of benzo[1,2-c:4,5-c']difuran as a bisdiene for the synthesis of fully conjugated ladder polymers
In parallel with the synthesis of fully conjugated ladder polymers using Cp-containing monomers, Blatter and Schlüter85 and Schlüter and co-workers86,87 developed another method, reacting highly reactive benzo[1,2-c:4,5-c']difuran 44 with bisalkene 33, 45 and 46 for the synthesis of ladder polymers 47, 48 and 49, respectively, via D–A polymerization (Schemes 7,9 and 10). The bisdiene 44, with its open-shell biradical character, was very unstable and extremely reactive and could not be isolated. However, Hart and Luo88 discovered that by heating the pentacene analog 43 above 190 °C, it was possible to generate 44 in situ via a twofold retro-D–A reaction (Scheme 9), and 44 could then be reacted with another dienophile.
The synthetic design of ladder polymers using 44 was similar to that of polymer 42. D–A polymerization was thus used for the one-pot construction of soluble kinked polymer precursors, which could be processed from solution, characterized and further ‘planarized’ into fully conjugated ladder polymers. For example, the D–A polymerization of bisdiene 44a with phenacene 45a provided ladder polymer 47a, which features separate acene-like and phenacene segments, although with poor solubility. In contrast, the use of bisdienophile 44b with longer alkyl side chains afforded ladder polymer 47b, which exhibited sufficient solubility for further characterizations. Its DP was estimated to be 7.85 On the other hand, the soluble polyacene precursor 48 was synthesized from the D–A polymerization of 33 with in situ generated 44b, reaching a DP of 23. Thermogravimetric analysis suggested that polymer 48 was stable up to 300 °C.86 However, the planarization of polymers 47 and 48 toward fully conjugated ladder polymers has not been reported. With the same bridged alkyl chains as polymer 42, another ‘polyacene-like’ ladder polymer 49 was synthesized by the D–A polymerization of 44b with bisdienophile 46.87 The soluble fraction of ladder polymer 49 gave a DP up to 7, but ~70% of the product was insoluble. Further dehydration of the obtained polymer precursor 49 under acidic conditions yielded a barely soluble material, which ideally would possess the fully conjugated structure 50. The UV–vis absorption of the soluble fraction of this product extended to above 650 nm, which suggested the formation of a low-defect ladder polymer with extended conjugation, although the unambiguous elucidation of the structure as 50 remained elusive.87
Anthracene as the diene to provide poly(iptycene)s
Iptycene is a class of aromatic compounds composed of bicyclooctatriene-bridged arenes. The simplest example is triptycene 51 (Scheme 11). The incorporation of iptycenes into poly(p-phenyleneethynylene) could enhance its solubility and mechanical properties.89 Iptycenes are often synthesized from the D–A reactions of anthracenes and higher acenes with dienophiles.90 Swager and co-workers91,92 synthesized the ladder-type poly(iptycene) 55 via the D–A polymerization of the AB-type iptycene monomer 52, which contains anthracene moieties (Scheme 11). The polymerization of 52 in refluxing decaline failed, but polymerization occurred when the reaction was carried out under 8.9 kbar to afford 55. The addition of 5 mol% of the A2 and B3 crosslinkers 53 and 54, respectively, increased the DP from 19 to 60. When polymer 55 was dispersed in uniaxially stretch-aligned polyvinyl chloride (PVC) thin films, it tended to align such that the short axes of the anthracene (end)groups were parallel to the stretching direction, as illustrated in Figure 2. Taking into account that anthracene itself prefers to align with its long axis parallel to the stretching direction, this behavior of poly(iptycene) 55 was controlled by the rigid linear nonplanar architecture granted by the unique iptycene building blocks in the backbone. The perpendicular packing of the backbone of poly(iptycene) 55 and PVC chains hardly perturbed the anisotropic alignment of the PVC polymer matrix. This greatly reduced the local PVC density change and the force applied on poly(iptycene) chains upon stretching (Figure 2).
Schematic illustration of stretch-aligned polyvinyl chloride (PVC) threaded through polymer 55 and the resulting perpendicular orientation of the two polymers. Reprinted with permission from Thomas et al.91 Copyright 2005 American Chemical Society. A full color version of this figure is available at the Polymer Journal journal online.
Swager et al.92 also reported the AB-type monomer 56 without preinstalled iptycene structure. Thermal examination by differential scanning calorimetry and thermogravimetric analysis revealed that polymerization occurred at ~162–215 °C to afford poly(iptycene) precursor 57. A DP up to 40 and PDI of 2.2–3.6 of 57 could be achieved when the polymerization was performed at 10 kbar in THF at 145 °C. The poly(iptycene) precursor 57 was soluble in DCM, chloroform and THF, allowing its complete dehydration in acidic conditions to form poly(iptycene) 58, which was thermally stable up to 350 °C. With the alkoxy side chains and iptycene structures in the backbone, ladder polymer 58 was readily soluble in DCM, chloroform and THF. The high solubility of ladder polymers 55 and 58 is intriguing for polymers with such rigid backbones. The iptycene structure has not been introduced into ladder polymer backbones by other synthetic methods, demonstrating the versatility of D–A polymerization. However, iptycene units interrupt the conjugation along the backbone, which is reflected in large band gaps of over 3 and 4 eV for ladder polymers 55 and 58, respectively.
These examples leave no doubt that D–A polymerization is one of the most powerful methods for constructing ladder polymers in a one-pot approach. In particular, the synthesis of fully conjugated ladder polymers, which can be considered the narrowest GNRs (vide infra), has thus been addressed since as early as 1970, with more successful examples in the 1990s, well before the emergence of the graphene field. Although the reported characterization remained limited in those days, it could be interesting to revisit such ladder polymers with the present state-of-the-art theoretical and experimental characterization methods.
Synthesis of GNRs through D–A polymerization
In the past decade, the bottom-up chemical synthesis of GNRs has been intensively investigated and has provided efficient access to GNRs with atomic precision. This precision is crucial for the rational control of their electronic, optical and even magnetic properties for use in further applications in nanoelectronics, optolectronics and spintronics.26, 27 The bottom-up fabrication of GNRs could be achieved by both surface-assisted and solution-mediated oxidative cyclization of PPs with properly designed structures. While the scale of the GNRs synthesized by surface-assisted methods is often very limited, the solution-synthesis protocols are more promising approaches to realizing the large-scale fabrication and practical applications of GNRs. The solution protocols for GNRs typically begin with the Suzuki, Yamamoto or D–A polymerization of tailor-made monomers, giving rise to structurally well-defined PPs that are straightforwardly converted to GNRs by intramolecular oxidative cyclodehydrogenation.26, 27 Since its development by Scholl93 and Clar94, oxidative cyclodehydrogenation, namely, the Scholl reaction, has been demonstrated to be highly efficient for the synthesis of a wide variety of π-extended polycyclic aromatic hydrocarbons throughout the twentieth century95. We further developed this reaction and used it for the planarization of polymeric systems such as PPs, providing GNRs in high quality. Herein, we focus on the recent advances in D–A polymerization for GNR synthesis.
A2B2-type D–A polymerization toward the synthesis of GNRs
A2B2-type D–A polymerization using a bis-Cp and bisacetylene as the A2 and B2 monomers to obtain phenylated PP is illustrated in the second section (Table 1).47, 49 The PPs obtained by this method exhibited high solubility in common organic solvents because of their flexible and twisted geometry, enabling further solution-phase cyclodehydrogenation toward GNRs. In 2000, we undertook our first attempt to planarize soluble PP 7 (Table 1) through intramolecular oxidative cyclodehydrogenation with copper(II) trifluoromethane sulfonate and aluminum(III) chloride.96 The partially dehydrogenated polymer 59 was obtained, as demonstrated by infrared and Raman spectroscopy (Scheme 12). However, the insolubility of polymer 59 hindered its further characterization.
In 2003, we reacted 1,4-diethynyl-2,5-di(4'-tert-butylphenyl)benzene (60) with 4,4'-(1,4-phenylene)bis(2,3,5-triphenylcyclopenta-2,4-dien-1-one) (22) (Scheme 5) to achieve A2B2-type D–A polymerization, producing the tert-butyl-substituted PP 61 with a DP of 24 (Scheme 11).97 The planarization of polymer 61 was achieved by treatment with iron(III) chloride in nitromethane and DCM, giving GNR 62. Nevertheless, GNRs 59 and 62 are not linear but rather contain irregular ‘kinks’ because of the structural isomerization of their PP precursors. In addition, the lengths of these GNRs are limited because of the difficulty in perfectly controlling the stoichiometry of the two different monomers used for polymerization, which led to PP precursors with low DP. This limitation is detrimental for the fabrication of single-GNR-based devices.
AB-type D–A polymerization toward synthesis of GNRs
In comparison with the A2B2-type approach, AB-type D–A polymerization can exclude the issue of stoichiometry, leading to PPs with high DPs and thus producing longer GNRs. In 2014, we reported the first example of ‘cove’-type GNRs 65a and 65b fabricated by the AB-type D–A polymerization protocol, giving unprecedented lengths of up to 600 nm, as determined from the molecular weight of their alkylated PP precursors.98 In this work, the rationally designed AB-type monomers 63a and 63b, having a Cp as the conjugated diene and an ethynyl group as the dienophile, were used to perform D–A polymerization and produced the alkylated PP precursors 64a and 64b, respectively (Scheme 13a). The high efficiency of such AB-type D–A polymerization was demonstrated by the GPC analysis of precursor 64a, finding an unprecedentedly high Mn of 340 000 g mol−1, which corresponded to a DP as high as 473 and a PDI of 1.9. This DP was significantly larger than those of PP precursors produced by A2B2-type D–A polymerization and other metal-catalyzed coupling reactions such as the Suzuki and Yamamoto polymerizations. The high-molecular weight of 64a was also confirmed by photon-correlation spectroscopy.
The planarization of polymers 64a and 64b into the corresponding GNRs 65a and 65b, respectively, was performed by intramolecular oxidative cyclodehydrogenation with iron(III) chloride as the oxidant and Lewis acid. As shown in Scheme 13a, 64a and 64b contained numerous regioisomers because each step of the D–A cycloaddition of monomers as well as intermediate products has two possible orientations. Nonetheless, all of them could lead to the straight and structurally well-defined GNRs 65a and 65b, respectively, with a theoretically estimated width of 0.69–1.13 nm (Scheme 13). The high efficiency of cyclodehydrogenation was confirmed by Fourier-transform infrared spectroscopy, Raman, solid-state nuclear magnetic resonance and UV–vis spectroscopy, as well as scanning probe microscopy. The Raman spectrum displayed intense G and D peaks as well as a distinct peak at 235 cm−1 from the radial-breathing-like mode, indicating the high uniformity of the width of the obtained GNRs (Figure 3a). The photoluminescence spectrum of GNR 65a exhibited a broad emission peak at 695 nm with a large Stokes shift of ~135 nm, which might originate from excimer-like states in stacks of individual GNRs or from other aggregation-induced effects and is currently under further investigation (Figure 3b).99 The corresponding photoluminescence excitation was consistent with the absorption band, proving that the observed Stokes-shifted luminescence arose from the GNRs.99 The scanning tunneling microscopy of GNR 65a and atomic force microscopy (AFM) of GNR 65b revealed the formation of self-assembled monolayers of uniform GNRs on a graphite surface (Figures 3c and d). The optical properties of GNR 65a were further investigated by femtosecond transient absorption spectroscopy, revealing efficient exciton–exciton annihilation to form a biexciton with a high binding energy of ~250 meV as well as stimulated emission from the biexciton state.100
(a) Raman spectrum of graphene nanoribbon (GNR) 65a measured at 532 nm (2.33 eV) on a powder sample with laser power below 0.1 mW. Inset: A magnified area of the spectrum (black oblong, bottom left) displaying a peak from the radial-breathing-like mode (RBLM) at 235 cm−1. Reprinted with permission from Narita et al.98 Copyright 2013 Nature Publishing Group. (b) Ultraviolet–visible (UV–vis) absorption, photoluminescence and photoluminescence excitation spectra (triangles) of GNR 65a. Reprinted with permission from Zhao et al.99 Copyright 2017 Elsevier Ltd. (c) Scanning tunneling microscope image of GNR 65a on highly oriented pyrolytic graphite (HOPG) (dry film) reveals a well-organized self-assembled monolayer of straight and uniform nanoribbons of up to ~60 nm in length. Reprinted with permission from Narita et al.98 Copyright 2013 Nature Publishing Group. (d) Atomic force microscopy (AFM) phase image of GNR 65b on HOPG (dry film) reveals a highly organized self-assembled monolayer of straight and uniform nanoribbons of over 200 nm in length. Reprinted with permission from Narita et al.98 Copyright 2013 Nature Publishing Group.
For the fabrication of single-GNR-based devices, it is essential to deposit isolated GNRs, which was achieved by immersing alkyl-functionalized Si/SiO2 substrates in a dispersion of GNR 65a, revealing a length of over 500 nm upon visualization by AFM (Figure 4a).101, 102 The length distribution of GNR 65a observed by AFM was in good agreement with the molecular weight distribution of the corresponding alkylated PP precursor 64a based on GPC analysis.101 Such individual GNR strands could then be used to fabricate single-molecule devices exhibiting electrical conduction (Figure 4b).102 Moreover, thin-film devices based on GNR 65a were also fabricated by drop casting the GNR dispersion onto functionalized Si/SiO2 substrates. The conductivity could be significantly enhanced after annealing at 500 °C in H2/Ar gases, which removed the insulating alkyl chains from the GNR edges (Figure 4c). The applicability of such GNR films as NO2 gas sensors has been demonstrated, with limits of detection as low as 50 ppb (Figure 4d). GNR 65a dispersed in a water/surfactant mixture was also used to fabricate FETs based on isolated GNR strands. Assuming that the channel width of the transistor was identical to the height (2 nm) of an individual GNR, a current density as high as 35 mA μm−1 and a field-effect mobility of 500 cm2 V−1 s−1 were demonstrated.103 Nevertheless, the on–off ratio was still very small, presumably due to the unintentional stacking of GNRs, which was theoretically shown to reduce the band gap. The on–off ratio was recently improved by using electrospray ionization deposition as well as graphene-based electrodes instead of metal electrodes to establish better contact with the GNRs.104 The resulting FET device exhibited n-type semiconducting behavior with electron mobility ranging from 10−3 to 10−2 cm2 V−1 s−1. However, the device mobility of GNR-based transistors remains underestimated due to the dominant contact resistance. In addition, on-surface synthesized GNRs have been investigated as semiconductor materials in FET devices, displaying higher on–off ratios than those of FETs based on solution synthesized GNRs. For example, Bokor and co-workers105 recently demonstrated high-performance FETs with on-surface derived GNRs, presenting a high on-current of ~1 μA and an on–off ratio of ~105. More recently, we reported a structurally defined GNR constructed by ambient-pressure chemical vapor deposition. The resulting FET device based on this GNR also exhibited a high on–off ratio.106 However, further efforts and improvements in deposition and device fabrication are required for achieving devices with better performance as well as for studying the intrinsic electronic properties of the GNRs in the device configurations.
(a) Atomic force microscopy (AFM) tapping-mode height image revealing synthesized graphene nanoribbon (GNR) 65a length >500 nm. (b) Current vs drain voltage (I – Vd) characteristic of an individual GNR 65a device. Inset shows a scanning electron microscopy image of a 20 nm gap between Ti/Pd and angle-deposited Pd. (c) Current vs drain voltage (I–Vd) of a typical GNR 65a film device under different gate voltages (Vgs). (d) Time domain-normalized conductance (G/G0) of the GNR 65a film device during the introduction of different concentrations of NO2. Green arrows correspond to the device being exposed to a certain concentration of NO2, whereas red arrows correspond to the device being flushed with Ar only. Reprinted with permission from Konnerth et al.101 Copyright 2014 American Chemical Society.
On the other hand, the functionalization of the edges of GNR 65 has been investigated as a way to tune the electronic and optical properties, as well as to confer other functions on the GNRs. We have demonstrated the edge chlorination of tert-butyl-substituted GNR 65c, leading to chlorinated GNR 66 with redshifted absorption and an optical band gap 0.2 eV lower (Scheme 13b).107 More recently, Fischer and co-workers108 synthesized GNR 65d bearing methyl esters and prepared composites with gold nanoparticles, which showed enhanced catalytic performance for the electrocatalytic reduction of CO2. This enhanced performance was probably because the GNR matrix effectively prevented the assembly of gold nanoparticles into larger aggregates.
To further modulate the optoelectronic properties of the GNRs, it is essential to change their widths and edge structures. We have achieved the lateral extension of GNR 65 by using the lateral extended monomer 67 with four extra benzene rings instead of monomer 63,109 following the same sequence of AB-type D–A polymerization and intramolecular cyclodehydrogenation (Figure 5a).3 The resulting GNR 68 exhibited a broader absorption pattern and a lowered optical band gap of ~1.2 eV compared to ~1.9 eV for the narrower GNR 65, demonstrating a width-dependent bandgap modulation (Figure 5b). We have more recently synthesized two GNRs (69 and 70) with the same edge structure as GNR 65 and intermediate widths between 65 and 68 (Scheme 14). GNR 69, with dodecyl chains attached to the outermost positions, has a planar conjugated structure, whereas 70, with the dodecyl chains at the innermost positions, possesses a distorted geometry based on theoretical studies. The structural distortion gave GNR 70 a lower band gap than that of GNR 69, as observed from their absorption spectra, accomplishing further bandgap tuning. The Raman spectra of GNRs 65, 68 and 69 displayed shifts of the low-energy radial-breathing-like mode peak from 130 to 230 cm−1, as predicted by theoretical calculations.110 Moreover, the photoconductivities of GNRs 60, 69 and 70 were compared using time-resolved terahertz spectroscopy, which suggested that the structural distortion by alkyl chain substitution has little effect on the photoconductive properties, while the width and edge structures of GNRs have a more significant role.111
(a) Synthesis of graphene nanoribbons (GNRs) 68 through AB-type Diels–Alder (D–A) polymerization of monomer 67. (b) Ultraviolet–visible–near Infrared (UV–vis–NIR) absorption spectra of GNRs 65a and 68 in THF. Inset: Photographs of dispersions of GNRs 65a and 68 in THF. Reprinted with permission from Narita et al.109 Copyright 2014, American Chemical Society.
Recently, Gorodetsky and co-workers112 proposed a novel AB-type aza-D–A (Povarov) polymerization method toward the synthesis of a nitrogen-doped N=7 armchair GNR 72 (Scheme 15). Monomer precursor 71, which was furnished with an N-naphthalenylmethanimine as the diene and an ethynyl group as the dienophile, was used for aza-D–A polymerization to give polybenzoquinoline 72. Polymer 72 exhibited a DP of up to 43 and a PDI of 1.4, based on GPC analysis. Polybenzoquinoline 72 could potentially be used as precursor for GNR 73, although the further planarization of 72 was not described.
In addition to the present D–A polymerization protocols, metal-catalyzed polymerization approaches have also been used for the construction of GNRs, including the A2B2-type113, 114, 115, 116 and AB-type117, 118 Suzuki polymerization as well as AA-type Yamamoto polymerization.119, 120, 121 Recently, Rubin and co-workers122 developed a new synthetic approach to GNRs through the topochemical polymerization of butadiyne-containing monomers followed by aromatization at high temperature. However, all these synthesis concepts except AB-type D–A polymerization could provide only GNRs with lengths limited to dozens of nanometers, which still needed further efforts.
Conclusion and outlook
The classical D–A reaction is still attracting attention from contemporary molecular architects in the field of synthetic polymer chemistry and is gaining increasing importance. The development of A2B2-type D–A polymerization using cyclopentadienones gave rise to a class of functional phenylated PP materials. With their high DP, these PPs exhibited promising applications as polymer membranes, especially as PEMs. These polymer membranes displayed comparable and sometimes even superior performance to that of the cutting-edge materials applied in gas separation, anionic/cationic fuel cells, batteries and electrodialysis. However, more efforts should still be made to reduce the water uptake and increase the proton conductivity and device durability of the PEMs of PPs, as well as to achieve a better understanding of their structure–property relationships. D–A reactions could also be used to synthesize hyperbranched PPs. In contrast to molecularly defined PP dendrimers synthesized step-by-step, hyperbranched PPs synthesized through uncontrolled one-pot D–A polymerization could provide three-dimensional macromolecular architectures with higher simplicity and efficiency, albeit compromised structural precision. The degree of branching of these hyperbranched PPs could be controlled by adjusting the stoichiometry of the different monomeric building blocks. To incorporate these hyperbranched PPs as well as phenylated PPs synthesized via other protocols, such as AB-type D–A polymerization, into the development of functional PPs with new architectures would reinforce the PP-based polymer membrane applications that have thrived in the past decade.
The D–A reaction has had a crucial role in the history of ladder polymer synthesis. D–A polymerization, especially using 1,2,5,6-tetramethylenecyclooctane and 1,2,4,5-tetramethylenecyclohexane, cyclopentadienone, benzo-[1,2-c:4,5-c′]difuran and anthracene as dienes, has allowed the synthesis of ladder polymers involving unique structural features such as rigid iptycene and semiflexible cyclooctadiene. The semiflexible ladder polymers containing cyclooctadiene confined the complicated three-dimensional motion of the polymer chains to two dimensions. Such a design would be interesting for fundamental studies of polymer folding and self-assembly. Moreover, fully conjugated ladder-type poly(fluoranthene) with similar structure to the belt region of C60 was also achieved by D–A polymerization, showing extension of optical absorption above 600 nm, which suggested a long effective conjugation length. These examples demonstrated that the D–A reaction is undoubtedly one of the most powerful reactions for constructing ladder polymers in one-pot polymerization. However, the requirement for one-pot polymerization renders the monomer structures more complicated and difficult to access, which seems to be the reason for the limited number of new examples in the past decade. With the emerging field of GNRs, ladder polymers, especially fully conjugated ladder polymers as the narrowest GNRs, could attract renewed attention in chemistry as well as in physics.
Finally, D–A polymerization offers a promising approach toward high-DP PP precursors for bottom-up GNR synthesis. In particular, AB-type D–A polymerization furnished GNRs with an unprecedented length of over 600 nm, which surpassed GNRs constructed by other reactions, including metal-catalyzed aryl–aryl coupling, which typically possess lengths up to ~100 nm. Furthermore, the width of the GNRs can be modulated by expanding the monomer structure, allowing tuning of their optoelectronic properties. Nevertheless, successful GNR synthesis through the AB-type D–A reaction is thus far limited to monomers based on ethynyl-substituted Cps. Therefore, the next challenge should be the design of new AB-type monomers equipped with different dienes and dienophiles for synthesizing a greater variety of GNRs with unique structures, heteroatom doping and/or added functional groups, while preserving the length exceeding 100 nm. It is especially desirable to synthesize low-bandgap GNRs with infrared absorption without broadening the ribbons, thus maintaining the high processability, which might be possible by incorporating zigzag and/or cove edges or heteroatom dopants. Such synthetic advances would be a key to enriching the GNR family and paving the way toward their future applications in nanoelectronics and optoelectronics.

Structures of poly(para-phenylene) 1, ladder-type poly(para-phenylene) 2, angular polyacene ladder polymer 3 and armchair graphene nanoribbon 4. Side chains are not shown.

Diels–Alder (D–A) reaction of (a) o-quinodimethane and N-phenylmaleimide and (b) 2,3,4,5-tetraphenylcyclopentadienone and diphenylacetylene.

Postfunctionalization of polyphenylenes (PPs) 7, 13 and 14 toward hydrocarbon-based polyelectrolytes 15–17.

Diels–Alder (D–A) polymerization of presulfonated 4,4'-(1,4-phenylene)bis- (2,3,5-triphenylcyclopenta-2,4-dien-1-one) monomer 18 toward polyelectrolyte 19a–c.

Structures of AB2-, AB- and A2-type tetraphenylcyclopentadienone-based monomers and B3- and B6-type multiacetylene monomers as building blocks for hyperbranched polyphenylenes (PPs).

Synthesis of the first Diels–Alder (D–A) ladder polymer 28.

Bisdiene and bisdienophile monomers featuring 1,2,5,6-tetramethylenecyclooctane, 1,2,4,5-tetramethylenecyclohexane and 1,4,5,8-tetrahydro-1,4:5,8-diepoxyanthracene structures and the resulting ladder polymers.

Synthesis of fully conjugated fluoranthene ladder polymers 39 and 42 by Diels–Alder (D–A) polymerization of AB-type monomers 38 and 40, respectively.

In situ generation of reactive benzo[1,2-c:4,5-c']difuran 44.

Ladder polymers 47–50 synthesized through Diels–Alder (D–A) polymerization using benzo[1,2-c:4,5-c']difuran.

Structures of iptycene-containing ladder polymers and the monomers from which they were derived.

Synthesis of graphene nanoribbons (GNRs) 59 and 62 through A2B2-type Diels–Alder (D–A) polymerization.

(a) Synthesis of graphene nanoribbon (GNR) 65 through AB-type Diels–Alder (D–A) polymerization. (b) Chlorination of GNR 65c to chlorinated GNR 66.

Structures of edge- and cove-substituted graphene nanoribbons (GNRs) 69 and 70.

Proposed synthesis of nitrogen-doped graphene nanoribbon (GNR) 73 through AB-type aza-D–A polymerization. D–A, Diels–Alder.
References
Diels, O. & Alder, K. Synthesen in der hydroaromatischen Reihe. Justus Liebig's Ann. Chem. 460, 98–122 (1928).
Nicolaou, K. C., Snyder, S. A., Montagnon, T. & Vassilikogiannakis, G. The Diels–Alder reaction in total synthesis. Angew. Chem. Int. Ed. 41, 1668–1698 (2002).
Ramdas, M. R., Kumar, K. S. S. & Nair, C. P. R. Click polymerizations: encouraging route for shape memory polymers. Mater. Lett. 172, 216–221 (2016).
Knall, A. C. & Slugovc, C. Inverse electron demand Diels–Alder (iEDDA)-initiated conjugation: a (high) potential click chemistry scheme. Chem. Soc. Rev 42, 5131–5142 (2013).
Nandivada, H., Jiang, X. & Lahann, J. Click chemistry: versatility and control in the hands of materials scientists. Adv. Mater. 19, 2197–2208 (2007).
Tasdelen, M. A. Diels–Alder ‘click’ reactions: recent applications in polymer and material science. Polym. Chem. 2, 2133 (2011).
Hizal, G., Tunca, U. & Sanyal, A. Discrete macromolecular constructs via the Diels–Alder ‘Click’ reaction. J. Polym. Sci. Part A 49, 4103–4120 (2011).
Kovacic, P. & Kyriakis, A. Polymerization of benzene top-polyphenyl by aluminum chloride-cupric chloride. J. Am. Chem. Soc. 85, 454–458 (1963).
Berresheim, A. J., Müller, M. & Müllen, K. Polyphenylene nanostructures. Chem. Rev. 99, 1747–1786 (1999).
Watson, M. D., Fechtenkötter, A. & Müllen, K. Big is beautiful—‘aromaticity’ revisited from the viewpoint of macromolecular and supramolecular benzene chemistry. Chem. Rev. 101, 1267–1300 (2001).
Hammer, B. A. G. & Müllen, K. Dimensional evolution of polyphenylenes: expanding in all directions. Chem. Rev 116, 2103–2140 (2016).
Bieri, M., Treier, M., Cai, J., Aït-Mansour, K., Ruffieux, P., Gröning, O., Gröning, P., Kastler, M., Rieger, R., Feng, X., Müllen, K. & Fasel, R. Porous graphenes: two-dimensional polymer synthesis with atomic precision. Chem. Commun. (Camb) 6919–6921 (2009).
Newby, J. J., Liu, C.-P., Müller, C. W., James, W. H., Buchanan, E. G., Lee, H. D. & Zwier, T. S. Spectroscopy and photophysics of structural isomers of naphthalene:z-phenylvinylacetylene. J. Phys. Chem. A 114, 3190–3198 (2010).
Türp, D., Nguyen, T.-T.-T., Baumgarten, M. & Müllen, K. Uniquely versatile: nano-site defined materials based on polyphenylene dendrimers. N. J. Chem. 36, 282–298 (2012).
Hammer, B. A., Moritz, R., Stangenberg, R., Baumgarten, M. & Müllen, K. The polar side of polyphenylene dendrimers. Chem. Soc. Rev. 44, 4072–4090 (2015).
Scherf, U. & Müllen, K. Polyarylenes and poly(arylenevinylenes), 7. A soluble ladder polymer via bridging of functionalized poly(p-phenylene)-precursors. Die Makromol. Chem. Rapid Commun. 12, 489–497 (1991).
Jones, R. G., Wilks, E. S., Metanomski, W. V., Kahovec, J., Hess, M., Stepto, R. & Kitayama, T. Compedium of Polymer Terminology and Nomenclature IUPAC Recommendations, (The Royal Society of Chemistry, Cambridge, UK, 2009).
Lee, J., Kalin, A. J., Yuan, T., Al-Hashimi, M. & Fang, L. Fully conjugated ladder polymers. Chem. Sci. 8, 2503–2521 (2017).
Grozema, F. C., van Duijnen, P. T., Berlin, Y. A., Ratner, M. A. & Siebbeles, L. D. A. Intramolecular charge transport along isolated chains of conjugated polymers: effect of torsional disorder and polymerization defects. J. Phys. Chem. B 106, 7791–7795 (2002).
Prins, P., Grozema, F. C., Schins, J. M., Patil, S., Scherf, U. & Siebbeles, L. D. A. High intrachain hole mobility on molecular wires of ladder-type poly(p-phenylenes). Phys. Rev. Lett. 96, 146601 (2006).
Samiullah, M., Moghe, D., Scherf, U. & Guha, S. Diffusion length of triplet excitons in organic semiconductors. Phys. Rev. B 82, 205211 (2010).
Grimsdale, A. C. & Müllen, K. Polyphenylene-type emissive materials: poly(para-phenylene)s. Polyfluorenes Ladder Polymers 199, 1–82 (2006).
Bheemireddy, S. R., Hautzinger, M. P., Li, T., Lee, B. & Plunkett, K. N. Conjugated ladder polymers by a cyclopentannulation polymerization. J. Am. Chem. Soc. 139, 5801–5807 (2017).
Scherf, U. Ladder-type materials. J. Mater. Chem. 9, 1853–1864 (1999).
Schlüter, A.-D. Ladder polymers: the new generation. Adv. Mater. 3, 282–291 (1991).
Narita, A., Wang, X. Y., Feng, X. & Müllen, K. New advances in nanographene chemistry. Chem. Soc. Rev. 44, 6616–6643 (2015).
Narita, A., Feng, X. & Müllen, K. Bottom-up synthesis of chemically precise graphene nanoribbons. Chem. Rec. 15, 295–309 (2015).
Chen, L., Hernandez, Y., Feng, X. & Müllen, K. From nanographene and graphene nanoribbons to graphene sheets: chemical synthesis. Angew. Chem. Int. Ed. 51, 7640–7654 (2012).
Schwierz, F. Graphene transistors. Nat. Nanotechnol. 5, 487–496 (2010).
Ritter, K. A. & Lyding, J. W. The influence of edge structure on the electronic properties of graphene quantum dots and nanoribbons. Nat. Mater. 8, 235–242 (2009).
Yazyev, O. V. A guide to the design of electronic properties of graphene nanoribbons. Accounts Chem. Res. 46, 2319–2328 (2013).
Li, X., Wang, X., Zhang, L., Lee, S. & Dai, H. Chemically derived, ultrasmooth graphene nanoribbon semiconductors. Science 319, 1229–1232 (2008).
Wang, X. R., Shi, Y. & Zhang, R. Field-effect transistors based on two-dimensional materials for logic applications. Chin. Phys. B 22, 098505 (2013).
Marmolejo-Tejada, J. M. & Velasco-Medina, J. Review on graphene nanoribbon devices for logic applications. Microelectron. J. 48, 18–38 (2016).
Osella, S., Narita, A., Schwab, M. G., Hernandez, Y., Feng, X., Müllen, K. & Beljonne, D. Graphene nanoribbons as low band gap donor materials for organic photovoltaics: quantum chemical aided design. ACS Nano 6, 5539–5548 (2012).
Villegas, C. E., Mendonca, P. B. & Rocha, A. R. Optical spectrum of bottom-up graphene nanoribbons: towards efficient atom-thick excitonic solar cells. Sci. Rep. UK 4, 6579 (2014).
Han, M. Y., Özyilmaz, B., Zhang, Y. & Kim, P. Energy band-gap engineering of graphene nanoribbons. Phys. Rev. Lett 98, 206805 (2007).
Chen, Z. H., Lin, Y. M., Rooks, M. J. & Avouris, P. Graphene nano-ribbon electronics. Phys. E 40, 228–232 (2007).
Tapasztó, L., Dobrik, G., Lambin, P. & Biró, L. P. Tailoring the atomic structure of graphene nanoribbons by scanning tunnelling microscope lithography. Nat. Nanotechnol. 3, 397–401 (2008).
Kosynkin, D. V., Higginbotham, A. L., Sinitskii, A., Lomeda, J. R., Dimiev, A., Price, B. K. & Tour, J. M. Longitudinal unzipping of carbon nanotubes to form graphene nanoribbons. Nature 458, 872–U875 (2009).
Jiao, L., Zhang, L., Wang, X., Diankov, G. & Dai, H. Narrow graphene nanoribbons from carbon nanotubes. Nature 458, 877–880 (2009).
Gandini, A. The furan/maleimide Diels–Alder reaction: a versatile click–unclick tool in macromolecular synthesis. Progr. Polym. Sci. 38, 1–29 (2013).
Cava, M. P. & Deana, A. A. Condensed cyclobutane aromatic compounds. VI. The pyrolysis of 1,3-dihydroisothianaphthene-2,2-dioxide: a new synthesis of benzocyclobutene1. J. Am. Chem. Soc. 81, 4266–4268 (1959).
Goodall, G. W. & Hayes, W. Advances in cycloaddition polymerizations. Chem. Soc. Rev. 35, 280–312 (2006).
Ried, W. & Bönnighausen, K. H. Diensynthesen mit Diinen. Chem. Beri. 93, 1769–1773 (1960).
Fieser, L. F. Hexaphenylbenzene. Org. Synth. 46, 44 (1966).
Stille, J. K., Harris, F. W., Rakutis, R. O. & Mukamal, H. Diels–Alder polymerizations: polymers containing controlled aromatic segments. J. Polym. Sci. Part B 4, 791–793 (1966).
Ried, W. & Freitag, D. Synthese von polyphenyl-poly-phenylen. Naturwissenschaften 53, 306–306 (1966).
Mukamal, H., Harris, F. W. & Stille, J. K. Diels–Alder polymers. III. Polymers containing phenylated phenylene units. J Polym Sci Part A-1 5, 2721–2729 (1967).
Stille, J. K., Rakutis, R. O., Mukamal, H. & Harris, F. W. Diels–Alder polymerizations. IV. Polymers containing short phenylene blocks connected by alkylene units. Macromolecules 1, 431–436 (1968).
Stille, J. K. & Noren, G. K. Diels–Alder polymers: polyphenylenes containing alternating phenylene and triphenylphenylene units (1). J. Polym. Sci. Part B 7, 525–527 (1969).
Wrasidlo, W. & Augl, J. M. Preparation of poly(octaphenyl-tetraphenylene). J. Polym. Sci. Part B 7, 519–523 (1969).
Speight, J. G., Kovacic, P. & Koch, F. W. Synthesis and properties of polyphenyls and polyphenylenes. J. Macromol. Sci. Part C 5, 295–386 (1971).
Noren, G. K. & Stille, J. K. Polyphenylenes. J. Polym. Sci. Macromol. Rev. 5, 385–430 (1971).
Kumar, U. & Neenan, T. X. Diels–Alder polymerization between bis(cyclopentadienones) and acetylenes. A versatile route to new highly aromatic polymers. Macromolecules 28, 124–130 (1995).
Suh, D., Jung, S.-H., Park, S.-J., Kim, D. & Cho, H.-N. Synthesis and properties of highly phenyl-substituted fluorene copolymers containing hole and electron transporting moieties via Diels–Alder Polymerization. Mol. Crystals Liquid Crystals 424, 159–165 (2004).
Kerry, F. G. Industrial Gas Handbook: Gas Separation and Purification, Taylor & Francis Group, LLC, Boca Raton, FL, USA, (2006).
Largier, T., Huang, F. & Cornelius, C. J. Homopolymer and multi-block Diels–Alder polyphenylenes: synthesis, physical properties, X-ray diffraction, and gas transport. Eur. Polym. J. 89, 301–310 (2017).
Bernardo, P., Drioli, E. & Golemme, G. Membrane gas separation: a review/state of the art. Ind. Eng. Chem. Res. 48, 4638–4663 (2009).
Wang, Y., Chen, K. S., Mishler, J., Cho, S. C. & Adroher, X. C. A review of polymer electrolyte membrane fuel cells: Technology, applications, and needs on fundamental research. Appl. Energy 88, 981–1007 (2011).
Kreuer, K. D. I. Conducting membranes for fuel cells and other electrochemical devices. Chem. Mater. 26, 361–380 (2014).
Hickner, M. A., Ghassemi, H., Kim, Y. S., Einsla, B. R. & McGrath, J. E. Alternative polymer systems for proton exchange membranes (PEMs). Chem. Rev. 104, 4587–4612 (2004).
Fujimoto, C. H., Hickner, M. A., Cornelius, C. J. & Loy, D. A. Ionomeric poly(phenylene) prepared by Diels−Alder polymerization: synthesis and physical properties of a novel polyelectrolyte. Macromolecules 38, 5010–5016 (2005).
Hickner, M. A., Fujimoto, C. H. & Cornelius, C. J. Transport in sulfonated poly(phenylene)s: proton conductivity, permeability, and the state of water. Polymer 47, 4238–4244 (2006).
Hibbs, M. R., Fujimoto, C. H. & Cornelius, C. J. Synthesis and characterization of poly(phenylene)-based anion exchange membranes for alkaline fuel cells. Macromolecules 42, 8316–8321 (2009).
Stanis, R. J., Yaklin, M. A., Cornelius, C. J., Takatera, T., Umemoto, A., Ambrosini, A. & Fujimoto, C. H. Evaluation of hydrogen and methanol fuel cell performance of sulfonated diels alder poly(phenylene) membranes. J. Power Sources 195, 104–110 (2010).
Largier, T. D., Wang, D., Mueller, J. & Cornelius, C. J. Improving electrodialysis based water desalination using a sulfonated Diels–Alder poly(phenylene). J. Membr. Sci. 531, 103–110 (2017).
Fujimoto, C., Kim, S., Stains, R., Wei, X. L., Li, L. Y. & Yang, Z. G. Vanadium redox flow battery efficiency and durability studies of sulfonated Diels–Alder poly(phenylene)s. Electrochem. Commun. 20, 48–51 (2012).
Lim, Y., Lee, H., Lee, S., Jang, H., Hossain, M. A., Cho, Y., Kim, T., Hong, Y. & Kim, W. Synthesis and properties of sulfonated poly(phenylene sulfone)s without ether linkage by Diels–Alder reaction for PEMFC application. Electrochim. Acta 119, 16–23 (2014).
Skalski, T. J., Britton, B., Peckham, T. J. & Holdcroft, S. Structurally-defined, sulfo-phenylated, oligophenylenes and polyphenylenes. J. Am. Chem. Soc. 137, 12223–12226 (2015).
Adamski, M., Skalski, T. J. G., Britton, B., Peckham, T. J., Metzler, L. & Holdcroft, S. Highly stable, low gas crossover, proton-conducting phenylated polyphenylenes. Angew. Chem. Int. Ed. 56, 9058–9061 (2017).
Voit, B. I. & Lederer, A. Hyperbranched and highly branched polymer architectures—synthetic strategies and major characterization aspects. Chem. Rev. 109, 5924–5973 (2009).
Morgenroth, F. & Müllen, K. Dendritic and hyperbranched polyphenylenes via a simple Diels–Alder route. Tetrahedron 53, 15349–15366 (1997).
Zhi, L., Wu, J., Li, J., Stepputat, M., Kolb, U. & Müllen, K. Diels–Alder reactions of tetraphenylcyclopentadienones in nanochannels: fabrication of nanotubes from hyperbranched polyphenylenes. Adv. Mater. 17, 1492–1496 (2005).
Stumpe, K., Komber, H. & Voit, B. I. Novel branched polyphenylenes based on A2/B3 and AB2/AB monomers via Diels–Alder cycloaddition. Macromol. Chem. Phys. 207, 1825–1833 (2006).
Kuchkina, N. V., Zinatullina, M. S., Serkova, E. S., Vlasov, P. S., Peregudov, A. S. & Shifrina, Z. B. Hyperbranched pyridylphenylene polymers based on the first-generation dendrimer as a multifunctional monomer. RSC Adv. 5, 99510–99516 (2015).
Bailey, W. J., Economy, J. & Hermes, M. E. Polymers. IV. Polymeric Diels–Alder reactions. J. Org. Chem. 27, 3295–3299 (1962).
Kohnke, F. H., Slawin, A. M. Z., Stoddart, J. F. & Williams, D. J. Molecular belts and collars in the making: a hexaepoxyoctacosahydro[12]cyclacene derivative. Angew. Chem. Int. Ed. 26, 892–894 (1987).
Wegener, S. & Müllen, K. New ladder polymers via repetitive Diels–Alder reaction under high pressure. Macromolecules 26, 3037–3040 (1993).
Wegener, S. & Müllen, K. 5,6,7,8-Tetramethylenebicyclo[2.2.2]oct-2-ene as ‘Bis(diene)’ in repetitive Diels–Alder reactions. Chem. Ber. 124, 2101–2103 (1991).
Pollmann, M., Wohlfarth, W., Müllen, K. & Lex, J. 1,2,5,6-Tetra-exo-methylenecynclooctane and [2.2]-(2,3)-furanophane as bis-diene components in Diels–Alder reactions. Tetrahedron Lett. 31, 2701–2704 (1990).
Pollmann, M. & Müllen, K. Semiflexible ribbon-type structures via repetitive Diels–Alder cycloaddition. Cage formation versus polymerization. J. Am. Chem. Soc. 116, 2318–2323 (1994).
Stille, J. K., Noren, G. K. & Green, L. Hydrocarbon ladder aromatics from a Diels–Alder reaction. J. Polym. Sci. Part A-1 8, 2245–2254 (1970).
Schlüter, A. D., Löffler, M. & Enkelmann, V. Synthesis of a fully unsaturated all-carbon ladder polymer. Nature 368, 831–834 (1994).
Blatter, K. & Schlüter, A. D. Ribbon-shaped structures via repetitive Diels–Alder reaction—a polycatafusene. Macromolecules 22, 3506–3508 (1989).
Vogel, T., Blatter, K. & Schlüter, A. D. A soluble polyacene precursor. Makromol. Chem. Rapid 10, 427–430 (1989).
Löffler, M., Schlüter, A. D., Gessler, K., Saenger, W., Toussaint, J. M. & Brédas, J. L. Synthesis of a fully unsaturated molecular board. Angew. Chem. Int. Ed. 33, 2209–2212 (1994).
Luo, J. & Hart, H. Bisannelation with a benzo[1,2-c:4,5-c']difuran equivalent: a new route to linear acene derivatives. J. Org. Chem. 53, 1341–1343 (1988).
Zhao, D. & Swager, T. M. Conjugated polymers containing large soluble diethynyl iptycenes. Org. Lett. 7, 4357–4360 (2005).
Hopf, H. Classics in Hydrocarbon Chemistry: Syntheses, Concepts, Perspectives, (Wiley-VCH, Weinheim, 2000).
Thomas, S. W. III, Long, T. M., Pate, B. D., Kline, S. R., Thomas, E. L. & Swager, T. M. perpendicular organization of macromolecules: synthesis and alignment studies of a soluble poly(iptycene). J. Am. Chem. Soc. 127, 17976–17977 (2005).
Chen, Z., Amara, J. P., Thomas, S. W. & Swager, T. M. Synthesis of a novel poly(iptycene) ladder polymer. Macromolecules 39, 3202–3209 (2006).
Scholl, R. & Mansfeld, J. meso-Benzdianthron (Helianthron),meso-Naphthodianthron, und ein neuer Weg zum Flavanthren. Ber. Dtsch. Chem. 43, 1734–1746 (1910).
Clar, E. & Ironside, C.T. Hexabenzocoronene. Proc. Chem. Soc. 150–150 (1958)
Grzybowski, M., Skonieczny, K., Butenschön, H. & Gryko, D.T. Comparison of oxidative aromatic coupling and the Scholl reaction. Angew. Chem. Int. Edit. 52, 9900–9930 (2013)
Shifrina, Z.B., Averina, M.S., Rusanov, A.L., Wagner, M. & Müllen, K. Branched polyphenylenes by repetitive Diels-Alder cycloaddition. Macromolecules 33, 3525–3529 (2000)
Wu, J. S., Gherghel, L., Watson, M. D., Li, J. X., Wang, Z. H., Simpson, C. D., Kolb, U. & Mullen, K. From branched polyphenylenes to graphite ribbons. Macromolecules 36, 7082–7089 (2003).
Narita, A., Feng, X., Hernandez, Y., Jensen, S. A., Bonn, M., Yang, H., Verzhbitskiy, I. A., Casiraghi, C., Hansen, M. R., Koch, A. H., Fytas, G., Ivasenko, O., Li, B., Mali, K. S., Balandina, T., Mahesh, S., De Feyter, S. & Mullen, K. Synthesis of structurally well-defined and liquid-phase-processable graphene nanoribbons. Nat. Chem. 6, 126–132 (2014).
Zhao, S., Rondin, L., Delport, G., Voisin, C., Beser, U., Hu, Y., Feng, X., Mullen, K., Narita, A., Campidelli, S. & Lauret, J. S. Fluorescence from graphene nanoribbons of well-defined structure. Carbon 119, 235–240 (2017).
Soavi, G., Dal Conte, S., Manzoni, C., Viola, D., Narita, A., Hu, Y., Feng, X., Hohenester, U., Molinari, E., Prezzi, D., Mullen, K. & Cerullo, G. Exciton–exciton annihilation and biexciton stimulated emission in graphene nanoribbons. Nat. Commun. 7, 11010 (2016).
Konnerth, R., Cervetti, C., Narita, A., Feng, X., Mullen, K., Hoyer, A., Burghard, M., Kern, K., Dressel, M. & Bogani, L. Tuning the deposition of molecular graphene nanoribbons by surface functionalization. Nanoscale 7, 12807–12811 (2015).
Abbas, A. N., Liu, G., Narita, A., Orosco, M., Feng, X., Mullen, K. & Zhou, C. Deposition, characterization, and thin-film-based chemical sensing of ultra-long chemically synthesized graphene nanoribbons. J. Am. Chem. Soc. 136, 7555–7558 (2014).
Zschieschang, U., Klauk, H., Mueller, I. B., Strudwick, A. J., Hintermann, T., Schwab, M. G., Narita, A., Feng, X. L., Muellen, K. & Weitz, R. T. Electrical characteristics of field-effect transistors based on chemically synthesized graphene nanoribbons. Adv. Electron Mater. 1, 1400010 (2015).
Fantuzzi, P., Martini, L., Candini, A., Corradini, V., del Pennino, U., Hu, Y., Feng, X., Mullen, K., Narita, A. & Affronte, M. Fabrication of three terminal devices by electrospray deposition of graphene nanoribbons. Carbon 104, 112–118 (2016).
Llinas, J. P., Fairbrother, A., Borin Barin, G., Shi, W., Lee, K., Wu, S., Yong Choi, B., Braganza, R., Lear, J., Kau, N., Choi, W., Chen, C., Pedramrazi, Z., Dumslaff, T., Narita, A., Feng, X., Müllen, K., Fischer, F., Zettl, A., Ruffieux, P., Yablonovitch, E., Crommie, M., Fasel, R. & Bokor, J. Short-channel field-effect transistors with 9-atom and 13-atom wide graphene nanoribbons. Nat. Commun. 8, 633 (2017).
Chen, Z. P., Zhang, W., Palma, C. A., Rizzini, A. L., Liu, B. L., Abbas, A., Richter, N., Martini, L., Wang, X. Y., Cavani, N., Lu, H., Mishra, N., Coletti, C., Berger, R., Klappenberger, F., Klaui, M., Candini, A., Affronte, M., Zhou, C. W., De Renzi, V., del Pennino, U., Barth, J. V., Rader, H. J., Narita, A., Feng, X. L. & Mullen, K. Synthesis of graphene nanoribbons by ambient-pressure chemical vapor deposition and device integration. J. Am. Chem. Soc. 138, 15488–15496 (2016).
Tan, Y. Z., Yang, B., Parvez, K., Narita, A., Osella, S., Beljonne, D., Feng, X. & Mullen, K. Atomically precise edge chlorination of nanographenes and its application in graphene nanoribbons. Nat. Commun. 4, 2646 (2013).
Wu, M., Wang, J., Wu, Z. X., Xin, H. L. L. & Wang, D. L. Synergistic enhancement of nitrogen and sulfur co-doped graphene with carbon nanosphere insertion for the electrocatalytic oxygen reduction reaction. J. Mater. Chem. A 3, 7727–7731 (2015).
Narita, A., Verzhbitskiy, I. A., Frederickx, W., Mali, K. S., Jensen, S. A., Hansen, M. R., Bonn, M., De Feyter, S., Casiraghi, C., Feng, X. & Mullen, K. Bottom-up synthesis of liquid-phase-processable graphene nanoribbons with near-infrared absorption. Acs Nano 8, 11622–11630 (2014).
Verzhbitskiy, I. A., Corato, M. D., Ruini, A., Molinari, E., Narita, A., Hu, Y., Schwab, M. G., Bruna, M., Yoon, D., Milana, S., Feng, X., Mullen, K., Ferrari, A. C., Casiraghi, C. & Prezzi, D. Raman fingerprints of atomically precise graphene nanoribbons. Nano Lett 16, 3442–3447 (2016).
Ivanov, I., Hu, Y., Osella, S., Beser, U., Wang, H. I., Beljonne, D., Narita, A., Mullen, K., Turchinovich, D. & Bonn, M. Role of edge engineering in photoconductivity of graphene nanoribbons. J. Am. Chem. Soc. 139, 7982–7988 (2017).
Dibble, D. J., Park, Y. S., Mazaheripour, A., Umerani, M. J., Ziller, J. W. & Gorodetsky, A. A. Synthesis of polybenzoquinolines as precursors for nitrogen-doped graphene nanoribbons. Angew. Chem. Int. Ed. 54, 5883–5887 (2015).
Dossel, L., Gherghel, L., Feng, X. L. & Müllen, K. Graphene nanoribbons by chemists: nanometer-sized, soluble, and defect-free. Angew. Chem. Int. Ed. 50, 2540–2543 (2011).
Kim, K. T., Jung, J. W. & Jo, W. H. Synthesis of graphene nanoribbons with various widths and its application to thin-film transistor. Carbon 63, 202–209 (2013).
Kim, K. T., Lee, J. W. & Jo, W. H. Charge-transport tuning of solution-processable graphene nanoribbons by substitutional nitrogen doping. Macromol. Chem. Phys. 214, 2768–2773 (2013).
Schwab, M. G., Narita, A., Osella, S., Hu, Y. B., Maghsoumi, A., Mavrinsky, A., Pisula, W., Castiglioni, C., Tommasini, M., Beljonne, D., Feng, X. L. & Mullen, K. Bottom-up synthesis of necklace-like graphene nanoribbons. Chem. Asian J. 10, 2134–2138 (2015).
Li, G., Yoon, K. Y., Zhong, X. J., Zhu, X. Y. & Dong, G. B. Efficient bottom-up preparation of graphene nanoribbons by mild Suzuki–Miyaura polymerization of simple triaryl monomers. Chem. Eur. J. 22, 9116–9120 (2016).
Yang, W. L., Lucotti, A., Tommasini, M. & Chalifoux, W. A. Bottom-up synthesis of soluble and narrow graphene nanoribbons using alkyne benzannulations. J. Am. Chem. Soc. 138, 9137–9144 (2016).
Schwab, M. G., Narita, A., Hernandez, Y., Balandina, T., Mali, K. S., De Feyter, S., Feng, X. L. & Mullen, K. Structurally defined graphene nanoribbons with high lateral extension. J. Am. Chem. Soc. 134, 18169–18172 (2012).
El Gemayel, M., Narita, A., Dossel, L. F., Sundaram, R. S., Kiersnowski, A., Pisula, W., Hansen, M. R., Ferrari, A. C., Orgiu, E., Feng, X. L., Mullen, K. & Samor, P. Graphene nanoribbon blends with P3HT for organic electronics. Nanoscale 6, 6301–6314 (2014).
Vo, T. H., Shekhirev, M., Kunkel, D. A., Morton, M. D., Berglund, E., Kong, L. M., Wilson, P. M., Dowben, P. A., Enders, A. & Sinitskii, A. Large-scale solution synthesis of narrow graphene nanoribbons. Nat. Commun. 5, 3189 (2014).
Jordan, R. S., Wang, Y., McCurdy, R. D., Yeung, M. T., Marsh, K. L., Khan, S. I., Kaner, R. B. & Rubin, Y. Synthesis of graphene nanoribbons via the topochemical polymerization and subsequent aromatization of a diacetylene precursor. Chem 1, 78–90 (2016).
Acknowledgements
We acknowledge our distinguished partner groups and dedicated associates who enabled our contributions to the achievements described in this article. We are grateful for the financial support by EC through MoQuaS (Molecular Quantum Spintronics FP7-ICT-2013-10 610449), the Marie Curie ITN project ‘iSwitch’ (GA No. 642196), the Graphene Flagship, ERC-Adv.-Grant 267160 (NANOGRAPH), the Max-Planck Society, the Office of Naval Research BRC Program (molecular synthesis and characterization) and the DFG Priority Program SPP 1459. We are also thankful for fruitful collaborations with BASF SE, Ludwigshafen.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Hou, IY., Hu, Y., Narita, A. et al. Diels–Alder polymerization: a versatile synthetic method toward functional polyphenylenes, ladder polymers and graphene nanoribbons. Polym J 50, 3–20 (2018). https://doi.org/10.1038/pj.2017.69
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pj.2017.69
This article is cited by
-
Nanoribbons as advanced nanomaterials for facile detection and efficient removal of heavy metals: a comprehensive review
International Journal of Environmental Science and Technology (2024)
-
Method of preparation of alkylated 1,3-diphenylpropan-2-ones, the components for assembly of graphene nanostructures
Russian Chemical Bulletin (2020)
-
Polycyclic aromatic hydrocarbons in the graphene era
Science China Chemistry (2019)
-
Inducing defects in ordered mesoporous carbons via the block copolymer-templated high-temperature carbonization of nitrogen-containing polymeric precursors
Polymer Journal (2018)