Abstract
Polyrotaxanes (PRXs), comprising many cyclic molecules threaded onto a linear polymer chain capped with bulky stopper molecules, have attracted considerable attention in the design of biomaterials. If cleavable linkages are included between the axle polymer terminals and bulky stopper molecules, PRXs acquire a stimuli-responsive dissociation ability. Such stimuli-cleavable PRXs can dissociate into their constituent molecules in response to various chemical and physical stimuli, such as the reductive intracellular environment and the acidic lysosomal environment. In this focus review, the basic principle of stimuli-cleavable PRX design and characteristics of stimuli-cleavable PRXs as drug delivery carriers are described. Additionally, recent progress in the use of β-cyclodextrin-threaded stimuli-cleavable PRXs as a therapeutic agent for treating Niemann–Pick-type C (NPC) disease, a family of lysosomal storage disorders characterized by lysosomal accumulation of cholesterol, is described. The lysosomal release of threaded β-cyclodextrins from PRXs leads to the formation of an inclusion complex with the cholesterols that accumulate in NPC disease, promoting extracellular excretion of cholesterols. Interestingly, these stimuli-cleavable PRXs improve impaired autophagic functions in NPC disease in addition to reducing cholesterol. Therefore, stimuli-cleavable PRXs are promising candidates for the treatment of intractable metabolic disorders.
Similar content being viewed by others
Introduction
Supramolecular compounds have received considerable attention and three scientists, Felinga, Stoddart and Sovage, who work in the field of supramolecular chemistry were awarded the Nobel Prize in Chemistry in 2016 for the development of ‘molecular machines’.1 A rotaxane is a representative supramolecular compound composed of one or more cyclic molecules threaded onto an axle molecule.2 A unique characteristic of rotaxanes is that they exhibit sliding and rotational motion of the mechanically interlocked cyclic molecules along the axle molecule. Stoddart et al.3 have reported the regulation of the localization of the cyclic molecules on the axle molecules of rotaxanes by means of chemical and electrochemical stimuli, and these authors have proposed that rotaxanes may be useful as controllable molecular switches.
A polymeric rotaxane, generally referred to as a polyrotaxane (PRX), is composed of many cyclic molecules threaded onto a linear polymer chain capped with bulky stopper molecules, and PRXs have also attracted attention because of their unique interlocked architecture (Figure 1).4 Cyclic molecules and a linear polymer can combine in a complementary manner. Therefore, the resulting inclusion complex formation is strongly related to the cross-sectional area of the polymer chain and the cavity size of the cyclic molecules. To date, many combinations of cyclic molecules and linear polymers that can form polymeric inclusion complexes (pseudo-PRXs) have been reported.4, 5, 6, 7 The most commonly used PRXs and pseudo-PRXs are composed of α-cyclodextrins (α-CDs) as the cyclic molecules and poly(ethylene glycol) (PEG) as the axle polymer.8 In general, these PRXs are synthesized by mixing PEG and α-CDs in an aqueous medium, causing an inclusion complex to spontaneously form between many α-CDs and PEG, which then precipitates from the solution. By capping both terminals with bulky stopper molecules under heterogeneous conditions, PRXs can be obtained. Although the PEG axle chain has a flexible conformation in an aqueous medium, the threading of α-CDs onto the PEG chain increases the persistence length, resulting in the formation of a rigid backbone structure.9 Additionally, the threaded cyclic molecules can move along the polymer chains in the PRXs; this dynamic motion is an intrinsic property of the PRX architecture, similar to that of small-molecule rotaxanes.
One of the practical applications of PRXs is as ‘slide-ring gels’, in which the cyclic molecules in the PRXs act as movable crosslinking points.10, 11 PRX-containing hydrogels show unique stress–strain profiles and properties of extreme stretchability and toughness because of the molecular mobility of the crosslinking points.11 Our research group has focused on the unique interlocked architecture of PRXs and has demonstrated their applications as biomaterials, such as enhanced multivalent interactions with ligand-modified PRXs and the direction of stem cell differentiation via the molecular mobility of PRX-immobilized surfaces.12, 13, 14 The molecular mobility observed in the PRX architecture may provide new insight into the design of biomaterials. Another interesting function of PRXs is the stimuli-induced dissociation of the PRX structure.13, 14 In this focus review, the recent progress in the design of stimuli-cleavable PRXs and their potential applications as supramolecular drugs are described.
Design and applications of stimuli-cleavable PRXs
The threading cyclic molecules in PRXs are mechanically interlocked onto the axle polymer by bulky stopper molecules at the terminals of the axle polymer. There are no chemical linkages between the cyclic molecules and the axle polymer. Taking advantage of this structural characteristic, PRXs can be dissociated into their constituent molecules in response to chemical and physical stimuli.13, 15, 16, 17, 18 If cleavable linkages are installed between the axle polymer terminals and the bulky stopper molecules, PRXs acquire a stimuli-responsive dissociation characteristic (Figure 2a). To date, our group has reported a variety of stimuli-cleavable PRXs that possess cleavable linkers, such as hydrolysable ester linkages,15 reduction-cleavable disulfide linkages,16 acid-cleavable ketal linkages17 and photo-cleavable o-nitrobenzyl esters.18 These stimuli-cleavable PRXs are regarded as a new class of biodegradable polymers and may be useful as biomaterials and drug delivery systems. In general, a long period of time is required for the complete degradation of conventional biodegradable polymers such as polyesters and polycarbonates. By contrast, stimuli-cleavable PRXs can be rapidly dissociated into their constituent molecules (axle polymer and cyclic molecules), because the cleavage of a single cleavable site induces the dissociation of the entire supramolecular structure.
Taking advantage of the unique dissociation characteristics of stimuli-cleavable PRXs, we have demonstrated the advantage of using stimuli-cleavable PRXs for the intracellular delivery of biologically active biomacromolecules such as plasmid DNA,16 small interfering RNA19 and acidic proteins.20 To promote the cellular internalization of these biomacromolecules, positively charged amino groups were introduced at the threading α-CD moieties of the PRXs. These cationic PRXs form positively charged polyelectrolyte complexes with negatively charged biomacromolecules through electrostatic interactions. This complex formation enhances cellular internalization because the positive surface charge of the complexes promotes their interaction with the plasma membrane. However, polyelectrolyte complexation typically masks the biological activity of biomacromolecules. To overcome this limitation, we developed PRXs possessing reduction-cleavable disulfide linkages in the axle polymer and N,N-dimethylaminoethyl (DMAE) groups as a cationic moiety (DMAE-SS-PRX) (Figure 2b). Because the intracellular concentration of l-glutathione, a biological reductive compound that can cleave the disulfide linkages, is 3–4 orders of magnitude higher than the extracellular concentration, DMAE-SS-PRX can be selectively dissociated in the intracellular environment. DMAE-SS-PRX can form stable polyelectrolyte complexes with plasmid DNA, small interfering RNA and acidic proteins under physiological conditions. However, upon the addition of reductive compounds (e.g., d,l-dithiothreitol, l-glutathione), the DMAE-SS-PRX readily dissociates, releasing the plasmid DNA, small interfering RNA and acidic proteins to exhibit their inherent biological activities. Indeed, polyelectrolyte complexes between DMAE-SS-PRX and plasmid DNA show a gene expression efficiency in cultured cells is three to four orders of magnitude higher than that of non-cleavable DMAE-modified PRX (DMAE-PRX)-based complexes.16 Similarly, DMAE-SS-PRX/small interfering RNA complexes show significantly higher gene silencing effects in cultured cells, and polyelectrolyte complexes between DMAE-SS-PRX and β-galactosidase, an anionic enzyme with an isoelectric point of 4.6, also show higher enzymatic activity in cultured cells than non-cleavable DMAE-PRX.19, 20 These results suggest that intracellular dissociation in response to the reductive intracellular environment contributes to intracellular release and the subsequent expression of biological activity. Therefore, stimuli-cleavable PRXs are regarded as promising candidates for the intracellular delivery of anionic biomacromolecules.
Interaction between PRXs and biological components
Recently, β-CDs have attracted attention because of recent studies that have revealed that β-CDs act as an active pharmaceutical ingredient for the treatment of many diseases, such as Alzheimer disease,21 Niemann–Pick-type C (NPC) disease,22 age-related macular degeneration23 and atherosclerosis.24 Because β-CDs can form inclusion complexes with cholesterols, β-CDs interact with cholesterols and remove them from the plasma membrane.25, 26 This effect sometimes modulates the localization and function of transmembrane proteins, particularly those localized in the lipid raft domain. This cholesterol removal effect is considered to be related to the therapeutic effect of β-CDs. However, cholesterol removal with β-CDs destabilizes or disrupts the plasma membrane and shows a toxic effect. Additionally, the administration of β-CD derivatives can cause acute toxicity,27 lung toxicity28 and hearing loss.29 Therefore, the therapeutic application of β-CD derivatives is limited because of their toxic effects. This limitation is difficult to overcome because both the therapeutic and toxic effects of β-CDs are related to the inclusion of cholesterols in their cavities.
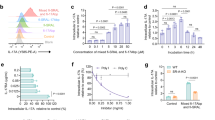
One strategy for overcoming the toxicity of β-CDs is to use the PRX structure. Because the cavities of β-CDs in PRXs are occupied by the polymer chain, β-CDs threaded into PRXs cannot form inclusion complexes with cholesterols, and PRXs show negligible interactions with cholesterols.30 Therefore, such PRXs are expected to show negligible toxicity. To validate this hypothesis, we designed β-CD-threaded PRXs composed of β-CDs modified with hydrophilic functional groups as the cyclic components, PEG-b-poly(propylene glycol)-b-PEG triblock copolymer (Pluronic P123) as the axle polymer (the β-CD forms inclusion complexes with the PEG-b-poly(propylene glycol) segments of the Pluronic31), and N-triphenylmethyl groups as stoppers (Figure 3a). These PRXs did not remove cholesterols from the plasma membranes of cultured fibroblasts, whereas plasma membrane cholesterols were removed into the culture medium upon treatment with 2-hydroxypropyl β-CDs (HP-β-CDs) (Figure 3b). The degree of membrane destabilization could be estimated based on the hemolysis of erythrocytes. The PRXs did not induce hemolysis, whereas the HP-β-CDs exhibited strong hemolysis at concentrations >5 mM (Figure 3c). Additionally, the PRXs did not reduce cell viability, even at a β-CD concentration of 20 mM, whereas cell viability was decreased for HP-β-CD concentrations >10 mM (Figure 3d). According to these results, it is concluded that the toxic effect of β-CDs can be masked by the formation of the supramolecular threaded PRX architecture.
(a) Chemical structure of β-cyclodextrin (β-CD)/Pluronic-based stimuli-cleavable polyrotaxanes (PRXs). (b) Amounts of cholesterol extracted from the plasma membranes of NPC1 fibroblasts treated with 2-hydroxypropyl β-CDs (HP-β-CDs) and PRXs at various concentrations for 2 h at 4 °C (n=3). (c) Hemolytic ratios of rat erythrocytes treated with HP-β-CDs and PRXs at various β-CD concentrations for 3 h (n=3). (d) Viability of NPC1 fibroblasts treated with HP-β-CDs and PRXs at various β-CD concentrations for 24 h (n=4). (e) Intracellular distribution analysis of FITC-HP-β-CDs (green) and FITC-PRXs (green) in NPC1 fibroblasts after incubation for 24 h (scale bar, 20 μm). Early endosomes, late endosomes and lysosomes were stained with EEA1, CD63 and LAMP1 antibodies, respectively (red). The nuclei were stained with DAPI (blue). (f) Colocalization percentage of FITC-HP-β-CD and FITC-PRXs with respect to early endosomes (EE), late endosomes (LE) and lysosomes (LY) after 24 h, as determined from confocal laser scanning microscopic (CLSM) images. The bars depict the mean values for 20 cells (****P<0.001). Reproduced with permission from ref. 30© 2014 Nature Publishing Group.
Another limitation regarding β-CDs is cellular internalization. Because β-CD derivatives strongly interact with plasma membrane cholesterols, the cellular internalization levels of β-CD derivatives are typically low. Therefore, β-CD derivatives do not strongly affect intracellular cholesterols; however, several diseases show abnormal intracellular cholesterol homeostasis. PRXs are expected to be internalized into cells through endocytosis, as the PRX architecture exhibits a negligible interaction with cholesterols in the plasma membrane. To confirm this hypothesis, the intracellular distributions of fluorescently labeled HP-β-CDs and PRXs were investigated.30 HP-β-CDs were detected at the cell surface, suggesting that HP-β-CDs localize at the plasma membrane and that colocalization with endosomes or lysosomes difficult to occur (Figures 3e and f). In contrast, PRXs were observed in the perinuclear regions of the cells and colocalized with late endosomes and lysosomes, indicating that PRXs are internalized into cells via endocytosis. To examine the detailed cellular internalization pathway of PRXs, the cellular internalization level of PRXs was assessed after treatment with pharmacological endocytosis inhibitors, such as dynasore (an inhibitor of dynamin, which blocks clathrin-mediated endocytosis), genistein (an inhibitor of several tyrosine kinases, which blocks caveolae-mediated endocytosis) and amiloride (an inhibitor of Na+/H+ exchange, which blocks micropinocytosis).32 The results showed that the cellular internalization level of the PRXs decreased for all tested endocytosis inhibitors, indicating that PRXs are internalized into cells via multiple endocytosis pathways. If PRXs are designed to dissociate under lysosomal acidic pH levels, threaded β-CDs may be locally released from the PRXs after endocytosis. This intracellular local release of β-CDs from PRXs is a potential approach for altering intracellular cholesterol level and localization.
Stimuli-cleavable PRXs for the treatment of Niemann–Pick-type C disease
NPC disease is a lysosomal storage disorder caused by a mutation in either the NPC1 or NPC2 protein.33, 34, 35 Both NPC1 and NPC2 have a pivotal role in the transport of low-density lipoprotein-derived cholesterols to the endoplasmic reticulum. If these proteins are deficient, low-density lipoprotein-derived cholesterols accumulate in lysosomes.33 As a result, patients suffering from this disease present fatal clinical symptoms such as progressive neurodegeneration and hepatosplenomegaly.33 Although an effective clinical treatment for NPC disease has not been established, HP-β-CDs have recently received attention because of their strong ability to promote the excretion of intracellular cholesterol in NPC disease cells.34 Consequently, the administration of HP-β-CDs significantly prolongs lifespan in mouse and feline models of NPC disease.21, 36, 37 Currently, clinical trials of the treatment of NPC disease with HP-β-CDs are underway.38 However, for the treatment of NPC disease, a high dose of HP-β-CDs is required in model mice (~4000 mg kg−1),21, 36 which may have severe adverse effects.
In this regard, stimuli-cleavable PRXs are potential candidates for overcoming the therapeutic and toxic effects of β-CD derivatives. Although PRXs themselves exhibit negligible interactions with cholesterol, stimuli-cleavable PRXs may dissociate in the intracellular environment and release β-CDs to exert a cholesterol-reducing effect in NPC disease. To confirm the cholesterol-reducing effect of stimuli-cleavable PRXs, intracellular cholesterol content in NPC disease patient-derived skin fibroblasts (NPC1 fibroblasts) was examined.30, 32, 39 In these experiments, β-CD-threaded PRXs composed of β-CDs modified with 2-(2-hydroxyethoxy)ethyl (HEE) groups as the cyclic components, Pluronic P123 as the axle polymer, N-triphenylmethyl groups as stopper molecules and reduction-cleavable disulfide linkages between the axle polymer terminals and stopper molecules (HEE-SS-PRXs) were prepared (Figure 3a). After the NPC1 fibroblasts were treated with HEE-SS-PRXs for 24 h, cholesterol content was assessed by means of filipin III staining, and the amount of total cholesterol was quantified using an enzymatic method. Figure 4a shows filipin III-stained normal and NPC1 fibroblasts, in which bright fluorescence was observed only in the NPC1 fibroblasts, indicating that excess cholesterol accumulated in the cells. The fluorescence intensity in the NPC1 fibroblasts treated with HP-β-CDs (1 mM) was slightly reduced. Interestingly, the fluorescence intensity in the NPC1 fibroblasts treated with HEE-SS-PRXs (1 mM threaded β-CDs) was reduced to the normal level. The quantification of total cholesterol revealed that the HEE-SS-PRXs reduced intracellular cholesterol to an ~100-fold lower concentration than did the HP-β-CDs (Figure 4b). These results indicate that the lysosomal release of threaded β-CDs from HEE-SS-PRXs causes significant cholesterol reduction. Note that the HEE-modified β-CDs and Pluronic P123 showed a lower capability for cholesterol reduction than did the HEE-SS-PRXs. Overall, the HEE-SS-PRXs reduced lysosomal cholesterol to a much lower concentration than did the HP-β-CDs without extracting cholesterol from the plasma membrane. The detailed mechanism involved in the reduction of lysosomal cholesterol remains unclear. Because the functions of cholesterol processing at the endoplasmic reticulum and cholesterol excretion via transporters in NPC1 cells are normal, it is considered that the β-CDs released from the PRXs form inclusion complexes with cholesterol in the lysosomes and assist in transporting cholesterol to the cytoplasm.40, 41
(a) Filipin staining for cholesterol in normal and NPC1 fibroblasts treated with 2-hydroxypropyl β-CDs (HP-β-CDs) (1 mM) and HEE-SS-PRXs (1 mM threaded β-CDs) for 24 h (scale bars, 20 μm). (b) Amounts of total cholesterol in normal and NPC1 fibroblasts treated with HP-β-CDs and HEE-SS-PRXs at various β-CD concentrations for 24 h (n=3) (*P<0.05, ***P<0.005). HEE, 2-(2-hydroxyethoxy)ethyl; PRX, polyrotaxane; SS, disulfide. Reproduced with permission from ref. 39© 2015 American Society for Biochemistry and Molecular Biology.
Effect of stimuli-cleavable PRXs on impaired autophagy in NPC disease
Autophagy is a system for the bulk degradation of cytoplasmic protein aggregates and subcellular organelles. In particular, basal autophagy has a pivotal role in the constitutive turnover of cytoplasmic components to maintain cellular function.42, 43 Atg5−/− or Atg7−/− mice display neurodegeneration and hepatomegaly, indicating that basal autophagy is strongly related to the pathology of neurodegeneration.44, 45 Indeed, impaired basal autophagy has been reported in various neurodegenerative diseases and lysosomal storage disorders.46, 47 Various studies have reported that the accumulation of autophagosomes occurs even under basal conditions in NPC disease.48, 49, 50 Therefore, improvement in impaired autophagy and lysosomal cholesterol accumulation is required for the treatment of NPC disease. Sarkar et al.50 have reported that HP-β-CDs prevent autophagic flux in normal and NPC1 model cells. Therefore, it remains challenging to improve lysosomal cholesterol accumulation and impaired autophagy in NPC disease.
Because of this, the effect of stimuli-cleavable PRXs on impaired autophagy in NPC disease was investigated. Figure 5a shows normal and NPC1 fibroblasts immunostained with anti-LC3, a marker protein for autophagosomes associated with the autophagosomal membrane.51 Consistent with the results of a previous report,50 a large number of LC3-positive puncta were observed in the NPC1 fibroblasts, even under basal conditions (cultured in normal growth medium) (Figure 5b). Although the treatment of NPC1 fibroblasts with HP-β-CDs (10 mM) markedly reduced cholesterol accumulation (Figure 4b), the number of LC3-positive puncta increased with the treatment of HP-β-CDs compared with untreated NPC1 fibroblasts, suggesting that β-CD derivatives perturb autophagic functions. In contrast, the number of LC3-positive puncta in NPC1 fibroblasts was reduced to the normal level upon treatment with HEE-SS-PRXs (1 mM threaded β-CDs) for 24 h.
(a) Immunostaining for LC3 in normal and NPC1 fibroblasts treated with 2-hydroxypropyl β-CDs (HP-β-CDs) (10 mM) and HEE-SS-PRXs (1 mM threaded β-CDs) for 24 h (scale bars, 20 μm). (b) Numbers of LC3-positive puncta in normal and NPC1 fibroblasts treated with HP-β-CDs (10 mM) and HEE-SS-PRXs (1 mM threaded β-CDs) for 24 h. The bars depict the mean values for 30 cells (*P<0.05, ****P<0.001). (c) Immunoblot analysis for LC3, p62 and β-actin in normal and NPC1 fibroblasts treated with HP-β-CDs (10 mM) and HEE-SS-PRXs (1 mM threaded β-CDs) for 24 h. (d) Confocal laser scanning microscopic (CLSM) images of normal and NPC1 fibroblasts transiently expressing mRFP-GFP tandem fluorescence-tagged LC3 (mRFP-GFP-LC3) (green and red puncta indicate GFP and mRFP, respectively) treated with HP-β-CDs (10 mM) and HEE-SS-PRXs (1 mM threaded β-CDs) for 24 h (scale bars, 20 μm). (e, f) Numbers of mRFP-positive/GFP-positive (e) and mRFP-positive/GFP-negative puncta (f) in normal and NPC1 fibroblasts expressing mRFP-GFP-LC3. The bars depict the mean values for 30 cells (*P<0.05, ***P<0.005). HEE, 2-(2-hydroxyethoxy)ethyl; PRX, polyrotaxane; SS, disulfide. Reproduced with permission from ref. 39© 2015 American Society for Biochemistry and Molecular Biology.
To further clarify the effect of HEE-SS-PRXs on impaired autophagy in NPC1 fibroblasts, the amounts of LC3-II and p62 (selectively degraded via autophagy52) were assessed by means of immunoblotting (Figure 5c). The intracellular levels of LC3-II and p62 in NPC1 fibroblasts were ~2-fold higher than those in normal fibroblasts. This is because of impaired proteolysis in the autolysosomes and/or impaired autophagic maturation (fusion of autophagosomes with lysosomes). HP-β-CD treatment significantly increased the levels of both LC3-II and p62 in NPC1 fibroblasts, suggesting that autophagic maturation was further perturbed. By contrast, both the LC3-II and p62 levels were decreased upon treatment with HEE-SS-PRXs, suggesting that the PRXs improved the impaired proteolysis in the autolysosomes and/or reduced the impairment of autophagic maturation.
To verify whether the HEE-SS-PRXs affected autolysosome formation in the NPC1 fibroblasts, the autophagic flux in the NPC1 fibroblasts was monitored using an expression vector encoding mRFP-GFP tandem fluorescence-tagged LC3 (mRFP-GFP-LC3) (Figure 5d).53 When mRFP-GFP-LC3 is localized to autophagosomes, both mRFP and GFP show fluorescence signals. By contrast, when mRFP-GFP-LC3 is localized to the acidic autolysosomes, only mRFP shows a fluorescence signal because GFP is quenched under acidic conditions. In the NPC1 fibroblasts, the number of mRFP-positive/GFP-positive puncta (autophagosomes) was increased and the number of mRFP-positive/GFP-negative red puncta (autolysosomes) was decreased compared with the normal fibroblasts (Figures 5e and f). The treatment of NPC1 fibroblasts with HP-β-CDs slightly increased the number of mRFP-positive/GFP-positive puncta, but a negligible change in the number of mRFP-positive/GFP-negative puncta was observed. Notably, the treatment of NPC1 fibroblasts with HEE-SS-PRXs markedly decreased the number of mRFP-positive/GFP-positive puncta and increased the number of mRFP-positive/GFP-negative puncta to levels comparable to those observed in normal fibroblasts.
Ballabio and co-workers54 have reported that lysosomal cholesterol accumulation inhibits the fusion of endosomes with lysosomes and of endosomes with autophagosomes in two cell models of lysosomal storage disorders. These authors showed that soluble N-ethylmaleimide-sensitive factor attachment protein receptors (SNAREs), such as VAMP7, Vit1b and syntaxin7, are preferentially sequestered to these cholesterol-enriched compartments. Sarkar et al. have reported that the fusion of syntaxin17 and VAMP8, essential factors in the fusion of autophagosomes with lysosomes55, 56, is altered by excess cholesterols, disrupting the formation of autolysosomes.50 Therefore, the reduction of lysosomal cholesterol caused by HEE-SS-PRXs is expected to improve the localization of these fusion factors. However, it remains unknown why treatment with β-CDs did not improve the impaired autophagic flux in NPC1 fibroblasts, although lysosomal cholesterol was reduced at a high concentration. The critical difference between HP-β-CDs and HEE-SS-PRXs is the ability to extract cholesterol from the plasma membrane (Figure 3b). It is predicted that the extraction of cholesterols from the plasma membrane by HP-β-CDs affects the process of autophagic flux.
Conclusion
In this focus review, the basic principles of stimuli-cleavable PRXs, the characteristics of stimuli-cleavable PRXs for drug delivery and therapeutic applications, and recent progress in the use of stimuli-cleavable PRXs as supramolecular therapeutic agents for treating NPC disease have been described. β-CD-threaded stimuli-cleavable PRXs are promising candidates for NPC disease therapy because of their excellent cholesterol-reducing effect and ability to improve the impaired autophagic flux in NPC disease. It will be important to evaluate the safety and therapeutic efficacy of stimuli-cleavable PRXs in a mouse model of NPC disease. Additionally, stimuli-cleavable PRXs may be useful for treating other diseases, such as other lysosomal storage disorders and hypercholesterolemia. We would like to pursue these possibilities along with developing an optimal design for PRXs as supramolecular therapeutic agents.
References
Van Noorden, R. & Castelvecchi, D. World’s tiniest machines win chemistry Nobel. Nature 538, 152–153 (2016).
Atkinson, I. M. & Lindoy, L. F. in Self Assembly in Supramolecular Systems (ed. Stoddart, J. F.) (The Royal Society of Chemistry, Cambridge, UK, 2000)
Bissell, R. A., Córdova, E., Kaifer, A. E. & Stoddart, J. F. A chemically and electrochemically switchable molecular shuttle. Nature 369, 133–137 (1994).
Harada, A., Takashima, Y. & Yamaguchi, H. Cyclodextrin-based supramolecular polymers. Chem. Soc. Rev. 38, 875–882 (2009).
Harada, A., Hashidzume, A., Yamaguchi, H. & Takashima, Y. Polymeric rotaxanes. Chem. Rev. 109, 5974–6023 (2009).
Wenz, G., Han, B.-H. & Müller, A. Cyclodextrin rotaxanes and polyrotaxanes. Chem. Rev. 106, 782–817 (2006).
Huang, F. & Gibson, H. W. Polypseudorotaxanes and polyrotaxanes. Prog. Polym. Sci. 30, 982–1018 (2005).
Harada, A., Li, J. & Kamachi, M. The molecular necklace: a rotaxane containing many threaded α-cyclodextrins. Nature 356, 325–327 (1992).
Yamada, S., Sanada, Y., Tamura, A., Yui, N. & Sakurai, K. Chain architecture and flexibility of α-cyclodextrin/PEG polyrotaxanes in dilute solutions. Polym. J. 47, 464–467 (2015).
Ito, K. Novel cross-linking concept of polymer network: synthesis, structure, and properties of slide-ring gels with freely movable junctions. Polym. J. 39, 489–499 (2007).
Imran, A. B., Esaki, K., Gotoh, H., Seki, T., Ito, K., Sakai, Y. & Takeoka, Y. Extremely stretchable thermosensitive hydrogels by introducing slide-ring polyrotaxane cross-linkers and ionic groups into the polymer network. Nat. Commun. 5, 5124 (2014).
Yui, N. & Ooya, T. Molecular mobility of interlocked structures exploiting new functions of advanced biomaterials. Chem. Eur. J. 12, 6730–6737 (2006).
Tamura, A. & Yui, N. Threaded macromolecules as a versatile framework for biomaterials. Chem. Commun. 50, 13433–13446 (2014).
Seo, J.-H., Kakinoki, S., Yamaoka, T. & Yui, N. Directing stem cell differentiation by changing the molecular mobility of supramolecular surfaces. Adv. Healthcare Mater. 4, 215–222 (2015).
Watanabe, J., Ooya, T. & Yui, N. Preparation and characterization of a polyrotaxane with non-enzymatically hydrolyzable stoppers. Chem. Lett. 27, 1031–1032 (1998).
Ooya, T., Choi, H. S., Yamashita, A., Yui, N., Sugaya, Y., Kano, A., Maruyama, A., Akita, H., Kogure, K. & Harashima, H. Biocleavable polyrotaxane–plasmid DNA polyplex for enhanced gene delivery. J. Am. Chem. Soc. 128, 3852–3853 (2006).
Nishida, K., Tamura, A. & Yui, N. Acid-labile polyrotaxane exerting endolysosomal pH-sensitive supramolecular dissociation for therapeutic applications. Polym. Chem. 6, 4040–4047 (2014).
Seo, J.-H., Fushimi, M., Matsui, N., Takagaki, T., Tagami, J. & Yui, N. UV-cleavable polyrotaxane cross-linker for modulating mechanical strength of photocurable resin plastics. ACS Macro 4, 1154–1157 (2015).
Tamura, A. & Yui, N. Cellular internalization and gene silencing of siRNA polyplexes by cytocleavable cationic polyrotaxanes with tailored rigid backbones. Biomaterials 34, 2480–2491 (2013).
Tamura, A., Ikeda, G., Seo, J.-H., Tsuchiya, K., Yajima, H., Sasaki, Y., Akiyoshi, K. & Yui, N. Molecular logistics using cytocleavable polyrotaxanes for the reactivation of enzymes delivered in living cells. Sci. Rep. 3, 2252 (2013).
Liu, B., Turley, S. D., Burns, D. K., Miller, A. M., Repa, J. J. & Dietschy, J. M. Reversal of defective lysosomal transport in NPC disease ameliorates liver dysfunction and neurodegeneration in the npc1−/− mouse. Proc. Natl Acad. Sci. USA 106, 2377–2382 (2009).
Yao, J., Ho, D., Calingasan, N. Y., Pipalia, N. H., Lin, M. T. & Beal, M. F. Neuroprotection by cyclodextrin in cell and mouse models of Alzheimer disease. J. Exp. Med. 209, 2501–2513 (2012).
Nociari, M. M., Lehmann, G. L., Perez Bay, A. E., Radu, R. A., Jiang, Z., Goicochea, S., Schreiner, R., Warren, J. D., Shan, J., Adam de Beaumais, S., Ménand, M., Sollogoub, M., Maxfield, F. R. & Rodriguez-Boulan, E. Beta cyclodextrins bind, stabilize, and remove lipofuscin bisretinoids from retinal pigment epithelium. Proc. Natl Acad. Sci. USA 111, E1402–E1408 (2014).
Zimmer, S., Grebe, A., Bakke, S. S., Bode, N., Halvorsen, B., Ulas, T., Skjelland, M., De Nardo, D., Labzin, L. I., Kerksiek, A., Hempel, C., Heneka, M. T., Hawxhurst, V., Fitzgerald, M. L., Trebicka, J., Björkhem, I., Gustafsson, J. Å., Westerterp, M., Tall, A. R., Wright, S. D., Espevik, T., Schultze, J. L., Nickenig, G., Lütjohann, D. & Latz, E. Cyclodextrin promotes atherosclerosis regression via macrophage reprogramming. Sci. Transl. Med. 8, 333ra50 (2016).
Kilsdonk, E. P., Yancey, P. G., Stoudt, G. W., Bangerter, F. W., Johnson, W. J., Phillips, M. C. & Rothblat, G. H. Cellular cholesterol efflux mediated by cyclodextrins. J. Biol. Chem. 270, 17250–17256 (1995).
Motoyama, K., Toyodome, H., Onodera, R., Irie, T., Hirayama, F., Uekama, K. & Arima, H. Involvement of lipid rafts of rabbit red blood cells in morphological changes induced by methylated β-cyclodextrins. Biol. Pharm. Bull. 32, 700–705 (2009).
Tanaka, Y., Ishitsuka, Y., Yamada, Y., Kondo, Y., Takeo, T., Nakagata, N., Higashi, T., Motoyama, K., Arima, H., Matsuo, M., Higaki, K., Ohno, K. & Irie, T. Influence of Npc1 genotype on the toxicity of hydroxypropyl-β-cyclodextrin, a potentially therapeutic agent, in Niemann–Pick Type C disease models. Mol. Genet. Metab. Rep. 1, 19–30 (2014).
Chien, Y. H., Shieh, Y. D., Yang, C. Y., Lee, N. C. & Hwu, W. L. Lung toxicity of hydroxypropyl-β-cyclodextrin infusion. Mol. Genet. Metab. 109, 231–232 (2013).
Crumling, M. A., Liu, L., Thomas, P. V., Benson, J., Kanicki, A., Kabara, L., Hälsey, K., Dolan, D. & Duncan, R. K. Hearing loss and hair cell death in mice given the cholesterol-chelating agent hydroxypropyl-β-cyclodextrin. PLoS ONE 7, e53280 (2012).
Tamura, A. & Yui, N. Lysosomal-specific cholesterol reduction by biocleavable polyrotaxanes for ameliorating Niemann–Pick type C disease. Sci. Rep. 4, 4356 (2014).
Fujita, H., Ooya, T., Kurisawa, M., Mori, H., Terano, M. & Yui, N. Thermally switchable polyrotaxane as a model of stimuli-responsive supramolecules for nano-scale devices. Macromol. Rapid Commun. 17, 509–515 (1996).
Tamura, A., Nishida, K. & Yui, N. Lysosomal pH-inducible supramolecular dissociation of polyrotaxanes possessing acid-labile N-triphenylmethyl end groups and their therapeutic potential for Niemann–Pick type C disease. Sci. Technol. Adv. Mater. 17, 361–374 (2016).
Vanier, M. T. Niemann–Pick disease type C. Orphanet J. Rare Dis. 5, 16 (2010).
Carstea, E. D., Morris, J. A., Coleman, K. G., Loftus, S. K., Zhang, D., Cummings, C., Gu, J., Rosenfeld, M. A., Pavan, W. J., Krizman, D. B., Nagle, J., Polymeropoulos, M. H., Sturley, S. L., Ioannou, Y. A., Higgins, M. E., Comly, M., Cooney, A., Brown, A., Kaneski, C. R., Blanchette-Mackie, E. J., Dwyer, N. K., Neufeld, E. B., Chang, T. Y., Liscum, L., Strauss, J. F. III, Ohno, K., Zeigler, M., Carmi, R., Sokol, J., Markie, D., O’Neill, R. R., van Diggelen, O. P., Elleder, M., Patterson, M. C., Brady, R. O., Vanier, M. T., Pentchev, P. G. & Tagle, D. A. Niemann pick C1 disease gene: homology to mediators of cholesterol homeostasis. Science 277, 228–231 (1997).
Naureckiene, S., Sleat, D. E., Lackland, H., Fensom, A., Vanier, M. T., Wattiaux, R., Jadot, M. & Lobel, P. Identification of HE1 as the second gene of Niemann–Pick C disease. Science 290, 2298–2301 (2000).
Davidson, C. D., Ali, N. F., Micsenyi, M. C., Stephney, G., Renault, S., Dobrenis, K., Ory, D. S., Vanier, M. T. & Walkley, S. U. Chronic cyclodextrin treatment of murine Niemann–Pick C disease ameliorates neuronal cholesterol and glycosphingolipid storage and disease progression. PLoS ONE 4, e6951 (2009).
Vite, C. H., Bagel, J. H., Swain, G. P., Prociuk, M., Sikora, T. U., Stein, V. M., O’Donnell, P., Ruane, T., Ward, S., Crooks, A., Li, S., Mauldin, E., Stellar, S., De Meulder, M., Kao, M. L., Ory, D. S., Davidson, C., Vanier, M. T. & Walkley, S. U. Intracisternal cyclodextrin prevents cerebellar dysfunction and Purkinje cell death in feline Niemann–Pick type C1 disease. Sci. Transl. Med. 7, 276ra26 (2015).
Matsuo, M., Togawa, M., Hirabaru, K., Mochinaga, S., Narita, A., Adachi, M., Egashira, M., Irie, T. & Ohno, K. Effects of cyclodextrin in two patients with Niemann–Pick Type C disease. Mol. Genet. Metab. 108, 76–81 (2013).
Tamura, A. & Yui, N. β-Cyclodextrin-threaded biocleavable polyrotaxanes ameliorate impaired autophagic flux in Niemann–Pick type C disease. J. Biol. Chem. 290, 9442–9454 (2015).
Peake, K. B. & Vance, J. E. Defective cholesterol trafficking in Niemann–Pick C-deficient cells. FEBS Lett. 584, 2731–2739 (2010).
Ramirez, C. M., Liu, B., Aqul, A., Taylor, A. M., Repa, J. J., Turley, S. D. & Dietschy, J. M. Quantitative role of LAL, NPC2, and NPC1 in lysosomal cholesterol processing defined by genetic and pharmacological manipulations. J. Lipid Res. 52, 688–698 (2011).
Mizushima, N., Levine, B., Cuervo, A. M. & Klionsky, D. J. Autophagy fights disease through cellular self-digestion. Nature 451, 1069–1075 (2008).
Shintani, T. & Klionsky, D. J. Autophagy in health and disease: a double-edged sword. Science 306, 990–995 (2004).
Hara, T., Nakamura, K., Matsui, M., Yamamoto, A., Nakahara, Y., Suzuki-Migishima, R., Yokoyama, M., Mishima, K., Saito, I., Okano, H. & Mizushima, N. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 441, 885–889 (2006).
Komatsu, M., Waguri, S., Chiba, T., Murata, S., Iwata, J., Tanida, I., Ueno, T., Koike, M., Uchiyama, Y., Kominami, E. & Tanaka, K. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 441, 880–884 (2006).
Nixon, R. A. The role of autophagy in neurodegenerative disease. Nat. Med. 19, 983–997 (2013).
Lieberman, A. P., Puertollano, R., Raben, N., Slaugenhaupt, S., Walkley, S. U. & Ballabio, A. Autophagy in lysosomal storage disorders. Autophagy 8, 719–730 (2012).
Liao, G., Yao, Y., Liu, J., Yu, Z., Cheung, S., Xie, A., Liang, X. & Bi, X. Cholesterol accumulation is associated with lysosomal dysfunction and autophagic stress in Npc1−/− mouse brain. Am. J. Pathol. 171, 962–975 (2007).
Elrick, M. J., Yu, T., Chung, C. & Lieberman, A. P. Impaired proteolysis underlies autophagic dysfunction in Niemann–Pick type C disease. Hum. Mol. Genet 15, 4876–4887 (2012).
Sarkar, S., Carroll, B., Buganim, Y., Maetzel, D., Ng, A. H., Cassady, J. P., Cohen, M. A., Chakraborty, S., Wang, H., Spooner, E., Ploegh, H., Gsponer, J., Korolchuk, V. I. & Jaenisch, R. Impaired autophagy in the lipid-storage disorder Niemann–Pick type C1 disease. Cell Rep. 5, 1302–1315 (2013).
Mizushima, N., Yoshimorim, T. & Levine, B. Methods in mammalian autophagy research. Cell 140, 313–326 (2010).
Bjørkøy, G., Lamark, T., Brech, A., Outzen, H., Perander, M., Overvatn, A., Stenmark, H. & Johansen, T. p62/SQSTM1 forms protein aggregates degraded by autophagy and has a protective effect on huntingtin-induced cell death. J. Cell Biol. 171, 603–614 (2005).
Kimura, S., Noda, T. & Yoshimori, T. Dissection of the autophagosome maturation process by a novel reporter protein, tandem fluorescent-tagged LC3. Autophagy 3, 452–460 (2007).
Fraldi, A., Annunziata, F., Lombardi, A., Kaiser, H. J., Medina, D. L., Spampanato, C., Fedele, A. O., Polishchuk, R., Sorrentino, N. C., Simons, K. & Ballabio, A. Lysosomal fusion and SNARE function are impaired by cholesterol accumulation in lysosomal storage disorders. EMBO J. 29, 3607–3620 (2010).
Itakura, E., Kishi-Itakura, C. & Mizushima, N. The hairpin-type tail-anchored SNARE syntaxin 17 targets to autophagosomes for fusion with endosomes/lysosomes. Cell 151, 1256–1269 (2012).
Furuta, N., Fujita, N., Noda, T., Yoshimori, T. & Amano, A. Combinational soluble N-ethylmaleimide-sensitive factor attachment protein receptor proteins VAMP8 and Vti1b mediate fusion of antimicrobial and canonical autophagosomes with lysosomes. Mol. Biol. Cell. 21, 1001–1010 (2010).
Acknowledgements
This work was financially supported by a Grant-in-Aid for Young Scientists (A) from the Japan Society for the Promotion of Science (JSPS) (No 16H05910, to AT); Grant-in-Aid for Young Scientists (B) from JSPS (No 26750155, to AT); Azuma Medical & Dental Research Grant (to AT).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Tamura, A., Yui, N. Rational design of stimuli-cleavable polyrotaxanes for therapeutic applications. Polym J 49, 527–534 (2017). https://doi.org/10.1038/pj.2017.17
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pj.2017.17
This article is cited by
-
Light-driven self-assembly of spiropyran-functionalized covalent organic framework
Nature Communications (2023)