Abstract
Artificial replication of the biomembrane systems in living organisms is attractive for the development of advanced functional materials but remains challenging for materials science because of the intricate function of these systems. To this end, free-standing polymeric ultrathin films (referred to as ‘polymer nanosheets’) have been developed as a structural analog of biomembranes, such as cellular membranes and basement membranes in an extracellular matrix, with a thickness of tens to hundreds of nanometers. In comparison with conventional plastic films, these ultrathin structures generate attractive properties for biomedical applications, including high flexibility and noncovalent adhesiveness. This report reviews the seminal features and characteristics of ‘nanosheet technology’, including fabrication methods, mechanical properties and biomedical and health-care applications (for example, wound dressings, tissue engineering materials and bioelectronic devices). Nanosheet technology is a promising approach for the development of advanced medical applications and health-care practices in surgery and regenerative medicine, as well as for connecting the human body to electronic interfaces for future medical applications.
Similar content being viewed by others
Introduction
Biomembranes are one of the most essential and common structures in our body and have important roles in maintaining homeostasis in biological systems, such as physical barriers isolating cells from the surrounding microenvironment, mechanical support for reinforcement of cellular structure, and mass transport including signal transduction between cells. Several recent efforts have attempted to replicate biomembrane structures using molecular assembly.1, 2 At the molecular scale, synthetic lipids were invented and used for the replication of cytoplasmic membranes (that is, phospholipid bilayer), and their assembled structures (for example, lipid bilayers and vesicular liposomes) were applied in the fields of biochemistry and biomedicine.3, 4 Beyond the molecular scale, the dynamic properties of biomembranes, such as flexibility and semipermeability, have also been the focus of recent studies in materials science.5, 6 Such unique structural properties originate from the ultrathin thickness and large lateral surface area of biomembranes. Synthetic ultrathin structures have the potential for use in the development of smart biomaterials and devices inspired by the functions of cytoplasmic membranes.
Polymeric ultrathin films (referred to as nanosheets and also known as nanofilms or nanomembranes) are a new class of polymeric nanomaterials, and their name originated from their ultrathin thickness (tens to hundreds of nanometers) and large surface area (several square centimeters).7 The distinguishing feature of these films is their free-standing structure, unencumbered by supporting substrates, which allows for expression of the dynamic properties of the polymer chains without restrictions from the substrates. Because of the huge size aspect ratio between the side length of the surface area and the thickness (that is, more than 106), unique interfacial and mechanical properties are obtained, including noncovalent adhesiveness, tunable flexibility and molecular permeability.8 These properties can also be controlled by the thickness. Their building blocks are chosen from various types of polymers including natural and synthetic polymers. In addition, the surface of the nanosheets can be tailored using drugs, fluorescent dyes, conductive polymers and even cells by integrating microfabrication or printing techniques, which broaden the applicability of these nanosheets for various medical fields. This review summarizes the recent progress in nanosheet technology that has been made by our group, and addresses fabrication methods based on molecular assembly, fundamental characteristics related to adhesive and mechanical properties and applications for biomedical and health-care fields (Figure 1). In particular, three topics are discussed (that is, surgical intervention, tissue engineering and health-care devices) that describe the practical application of nanosheets through the utilization of their unique properties.
Characteristics of polymer nanosheets and their biomedical applications. Partially reproduced from ref. 25. A full color version of this figure is available at Polymer Journal online.
Fabrication and fundamental properties of free-standing polymer nanosheets
Preparation of free-standing polymer nanosheets
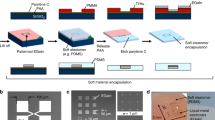
The basic methods used to detach ultrathin structures from a solid substrate (for example, glass substrates, silicon wafers, plastic films) can be categorized into two approaches as follows: ‘sacrificial’ layer method or ‘(water-soluble) supporting’ layer method (Figure 2a). In both the techniques, the appropriate ultrathin polymeric layer (constituent of the nanosheet) must be chosen while taking into account the surface wettability of the underlying layer. In the sacrificial layer method, the sacrificial layer (for example, cellulose acetate) is initially formed on the substrate, on which the nanosheet layer is prepared using conventional film preparation methods, such as dip-coating,9 spin-coating,10 layer-by-layer,11 Langmuir–Blodgett12 and sol–gel methods.13 Then, the sacrificial layer is dissolved in a specific solvent that does not dissolve the nanosheets, allowing for the detachment of the nanosheet from the substrate. In the supporting layer method, the nanosheet layer is directly prepared on the substrate. Then, the supporting layer (for example, polyvinyl alcohol (PVA)) is coated on the nanosheet. Because of the weak interactions between the nanosheet layer and the substrate, the nanosheet is detached from the substrate with the supporting layer. Finally, dissolution of the supporting layer releases the nanosheet with the free-standing structure. The nanosheets prepared using this method can be manipulated with the aid of a water-soluble supporting layer, allowing for the practical application of these nanosheets for ubiquitous transfer to desirable surfaces including biological tissues and organs.
(a) Schematic explanation of sacrificial and supporting layer methods for the preparation of free-standing polymer nanosheets (left), a macroscopic image of a 75-nm-thick polysaccharide nanosheet floating in phosphate-buffered saline (top right), and an scanning electron microscopic cross-sectional image of the 75-nm-thick polysaccharide nanosheet on an alumina porous membrane (bottom right). (b) Adhesion (inset: scratched surface of the nanosheets with different thicknesses) and (c) mechanical properties of the polysaccharide nanosheets. Partially reproduced from ref. 26.
In our group, biopolymers, such as chitosan (polycation) and sodium alginate (polyanion), were used to fabricate free-standing polysaccharide nanosheets for biomedical applications, such as wound dressings and artificial skins.14, 15 Each polysaccharide layer was assembled on a sacrificial layer (for example, cellulose acetate, photoresist) using a spin-coating-assisted layer-by-layer method, which allows the thickness to be controlled by the number of polysaccharide layer pairs. Then, the polysaccharide nanosheet was released in acetone by dissolution of the sacrificial layer, and the free-standing structure maintained the original size and shape of the substrate. Indeed, an atomic force microscopy scan revealed that the film thickness was 30.2±4.3 nm (10.5 layer pairs of polysaccharide) with a smooth and flat surface that had a root mean square roughness of 7.1±2.4 nm. It is important to note that polysaccharide nanosheets have also been fabricated according to the supporting layer method using a PVA supporting film, which is beneficial for surgical application of the nanosheets as wound-dressing materials.8
Biodegradable polylactides, such as poly(lactic acid) (PLA), have also been used as building blocks for nanosheets. In a similar manner to that used for the polysaccharide nanosheets, free-standing PLA nanosheets were fabricated using both the sacrificial layer and the supporting layer methods. Rather than using the layer-by-layer method, the nanosheets are prepared in a single-coating process, where the film thickness is controlled by the concentration of the PLA solution. Therefore, PLA is appropriate for large-scale manufacturing using an industrial printing process (for example, the roll-to-roll process). In fact, we recently succeeded in preparing a nanosheet on the meter length scale using the roll-to-roll process, which dramatically increases the production scale and potential application of these nanosheets.
Adhesive and mechanical properties of polymer nanosheets
For biomedical application of polymer nanosheets, two of the most important characteristics are flexibility and physical adhesiveness. Owing to the huge size aspect ratio, the nanosheets exhibit extremely high flexibility compared with that of micrometer-thick films. To evaluate the adhesive properties of nanosheets, the critical loading force for scratching the nanosheets off the substrates was measured in terms of the adhesive force using a micro-scratch test.16 We found that the critical load of the polysaccharide nanosheets on the SiO2 substrates substantially increased as the film thickness decreased below 200 nm (Figure 2b).
The mechanical properties of the polysaccharide nanosheets were also evaluated using the bulge test. The bulge test is based on the relationship between the mechanical pressure and shape deflection of the nanosheets.17, 18 For example, three types of polysaccharide nanosheets with different thicknesses (35, 75 and 114 nm) were physically adhered to steel plates with a 1-mm-diameter circular hole, and air pressure was applied to the adhered nanosheet through the hole. Nonlinear deflection occurred as the air pressure increased. Interestingly, the elastic modulus of the nanosheet decreased as the film thickness decreased (Figure 2c). However, the elastic modulus of the bulk is principally defined by the constituent materials. Therefore, the mechanical properties of the nanosheet are controlled by the film thickness in the region of tens to hundreds of nanometers thick. A similar trend in the mechanical properties was also observed for the poly(l-lactic acid) nanosheets when tested using the ‘strain-induced elastic buckling instability for mechanical measurement (SIEBIMM)’.19 In the SIEBIMM test, the buckling metrology between the nanosheets and elastic substrates (for example, polydimethylsiloxane (PDMS)) is used to measure the Young’s modulus. The Young’s modulus of the poly(l-lactic acid) nanosheets gradually increased with increasing film thickness20 and reached the bulk value of the poly(l-lactic acid) films.21 Overall, the thickness has a substantial effect on the mechanical properties of the polymer nanosheets and generates outstanding properties including better flexibility and physical adhesiveness compared with those of micrometers-thick polymer films.
Polymer nanosheets for surgical treatment
Ultrathin wound-dressing materials: nano-adhesive plasters
The surgical treatment of tissue injuries has been achieved using traditional techniques, such as suturing with threads, plication with a stapler and overlapping using wound-dressing materials. Although these surgical treatments are reliable for wound repair, several drawbacks remain, such as the time-consuming nature of the process, the decrease in organ function and the unwanted side effects derived from the materials (for example, postsurgical tissue adhesion). In particular, closing failure in thoracic surgery is sometimes lethal for patients because pulmonary air leakage substantially reduces the respiratory function and leads to traumatic complications. Therefore, overlapping treatment using wound-dressing materials is ideal for the treatment of air leakage because the process is straightforward and involves simply patching of the defect region without damaging or reducing the original volume of the pleural tissue.22, 23 For example, fibrin glue (sheet), which is composed of fibrin-glue-coated collagen fleece, has been used as a wound dressing.24 However, the use of fibrin materials sometimes leads to adverse events (for example, tissue adhesion between the defects and the intact chest wall).
Therefore, we focused on the surgical application of polymer nanosheets as alternatives to conventional dressing materials, and these nanosheets are referred to as ‘nano-adhesive plasters’. Ideally, the nanosheet is conformable to the pleural tissue and physically seals the defect portion like a plaster.25 In particular, the adhesive properties of the nanosheet will provide advantages for overlapping therapy to prevent postsurgical tissue adhesion and reduce the inflammatory response derived from the source materials.
To evaluate the effect of wound dressings using nanosheets, polysaccharide nanosheets were used for the treatment of visceral pleural defects.26 The pleural defects (size: 3.2 cm2) of beagle dogs were covered with a 75-nm-thick polysaccharide nanosheet, and their efficacy was compared with that of a dressing using conventional fibrin sheets (TachoComb). With the aid of a PVA supporting film (70 μm in thickness), the nanosheet was transferred to the defect region, which physically overlapped the defect after complete dissolution of the PVA. Three hours after the application, the nanosheet exhibited good mechanical stability against an applied pressure of approximately 57 cm H2O, which is comparable to the durability of fibrin sheets (Figure 3a). Moreover, histological images of the defect tissue treated with the polysaccharide nanosheet exhibited a minute inflammatory response on day 3 and no tissue adhesion on day 7. However, the defect tissue treated with the fibrin sheet induced inflammation on day 3 and strong tissue adhesion to the chest wall on day 7 owing to the fibrin glue (Figure 3b). Therefore, overlapping treatment using polysaccharide nanosheets exhibited significant advantages by maintaining the remaining function of the lung as well as reducing postsurgical tissue adhesion.
(a) Visceral pleural defect repair using polysaccharide nanosheets (arrows showing four corners of the nanosheet with a size of 2 × 2 cm). (b) Histological images of pleura tissue 7 days after treatment with the polysaccharide nanosheet and a fibrin sheet (hematoxylin–eosin staining, magnification × 4). (c) Antimicrobial properties of the polysaccharide nanosheets with or without tetracycline (TC) against Escherichia coli and their applications on perforated murine cecum (size of nanosheet: 2 × 2 cm) and burned murine dorsal skin (size of nanosheet: 2 × 2 cm, with two pieces; applied regions shown by dotted lines). Partially reproduced from refs 26, 29 and 30.
Antibacterial therapeutics using drug-loaded nanosheets
In contrast to repairing pleural defects, gastrointestinal surgery of perforated lesions has the potential risk of bacterial infection or even severe sepsis.27, 28 Spraying antibiotics is a surgical treatment used to fight this type of infection. However, the spraying method is sometimes insufficient because the perforated lesion is typically wet and friable. Therefore, we developed nanosheets loaded with antibiotics. These nanosheets were composed of a tetracycline layer sandwiched by a polysaccharide nanosheet and a poly(vinyl acetate) nanosheet.29 Owing to the semipermeable property of poly(vinyl acetate) (glass transition temperature (Tg): 32 °C), tetracycline was sustainably released from the nanosheet for up to 6 h, and an antimicrobial effect against Escherichia coli was observed (Figure 3c). The practical treatment of a gastrointestinal perforation was demonstrated by patching the antibiotic-loaded nanosheet to a murine cecum puncture with a lesion size of 0.8 mm2. The overlapping treatment using the antibiotic-loaded nanosheets exhibited 100% viability in murine after 7 days, and the treatment using the control pristine nanosheets and sham (no treatment) exhibited half and null, respectively. In addition, the bacterial number in the peritoneal lavage also decreased significantly by approximately 15 × 104-fold after treatment using antibiotic-loaded nanosheets compared with that using the control nanosheets. These results suggest that there are two distinct barrier effects that can be attributed to the nanosheet. The first effect involves a physical barrier caused by the nanosheet structure itself, and the second effect involves an antimicrobial barrier owing to the localized antibiotics. This study demonstrated that antibiotics as well as other drugs, such as silver nanoparticles, anticancer drugs and growth factors, would be good candidates for biological functionalization of the nanosheet.30, 31 Nanosheets are a promising platform for the management of the controlled release of drugs for advanced surgery.
Microfabricated nanosheets for tissue engineering
Free-standing nanosheets as synthetic scaffolds for cellular organization
Tissue engineering is expected to be a promising technology for future medical applications and contribute to regenerative medicine,32 in vitro drug-screening systems33 and bio-hybrid robotics.34 In tissue engineering, directing cellular organization using artificial scaffolds is an essential tool for engineering synthetic tissues.35 Many research studies have paved the way for creating functional scaffolds using nano- or microfabrication techniques, as exemplified by porous composites,36 self-organized microwrinkles,37 electrospun nanofibers,38 bioprinting39 and others.40 In particular, soft lithography using PDMS stamps broadened the potential applicability of printing technology for tissue engineering. For example, microcontact printing allows for precise patterning of cell-adhesive molecules (for example, collagen, fibronectin, laminin), which direct selective adhesion and differentiation of localized cells.41 Microgroove molding is also used for topographically directed cell alignment on microgrooved scaffolds.
Inspiration from natural tissue structures is important for designing biomaterials with smart surfaces for directing cellular organization (for example, anisotropic alignment of muscle fibers). Focusing on the microstructure of natural tissues, basement membranes in an extracellular matrix have an important role in organizing cells into tissues. The basement membrane is composed of a nanofibrous structure consisting of structural proteins and polysaccharides (for example, collagen, laminin, fibronectin, vitronectin, elastin, hyaluronan and chondroitin sulfate) with a thickness of tens to hundreds of nanometers.42, 43 On the basis of the dimensional features of the basement membrane, free-standing polymer nanosheets may replicate the properties of basement membranes for the design of synthetic scaffolds. Therefore, we envisioned the fabrication of ultrathin scaffolds that topographically mimic the microstructure of biological tissues using microfabrication techniques.
As a first attempt, we chose polystyrene, which is a conventional polymer used for cell culture dishes, as a building block for the nanosheets and modified the surface with fibronectin using microcontact printing. The geometrical features of the micropattern design are critically important for engineering tissue (for example, skeletal muscles consisting of myofibers).44 Therefore, a 50-μm stripe of a fibronectin micropattern was stamped on the nanosheet, on which skeletal myoblasts (C2C12) were cultured. The fibronectin micropatterns allowed for the anisotropic alignment of C2C12 on the nanosheet. To detach the nanosheet with aligned C2C12, a sacrificial layer method was used. Thermoresponsive poly(N-isopropylacrylamide) was coated between the glass substrate and the nanosheet. Because the water solubility of poly(N-isopropylacrylamide) changes at a lower critical solution temperature (32 °C), the nanosheet is stable during cell culture conditions at 37 °C (poly(N-isopropylacrylamide): hydrophobic) and detaches from the substrate at 4 °C (poly(N-isopropylacrylamide): hydrophilic). After the detachment process, the micropatterned cells were stable and maintained their aligned structure on the nanosheet (Figure 4a). Furthermore, owing to the mechanical reinforcement of the nanosheets, the cellular construct was easily manipulated with tweezers, allowing for three-dimensional engineering of muscle architecture by multilayering or rolling up around specific templates. We demonstrated the preparation of an artificial tubular structure using cell/nanosheet constructs by rolling the nanosheet with myoblasts around the template (for example, silicone tube, 3 mm diameter). The lateral image of the rolled myoblasts on the nanosheet showed a layered structure, and the cross-sectional image showed a tightly wrapped structure around the silicone tube. These tubular structures that consist of muscle cells are typically found in our blood vessels or intestinal tracts and exhibit specific biomechanical functions that originate from their structures (for example, controlled flux of blood or nutrients).45, 46
(a) Free-standing polystyrene nanosheets (left, with a size of 1 × 1 cm) with micropatterned C2C12 myoblasts (middle, blue: nucleus, red; F-actin) that were formed into a tubular structure (right indicated by an arrow). (b) Microgrooved nanosheets consisting of PLGA (poly(lactic-co-glycolic acid)) before (top left) and after (bottom left) the supporting PVA layer was dissolved that spatially coordinated the alignment of the C2C12 myoblasts (right, green: myosin heavy chain, blue: nucleus, red; F-actin). (c) Local delivery of RPE cells (green: RPE cells, red: nanosheet) to subretinal space using micropatterned nanosheets. Partially reproduced from refs 42, 47, 48, 51. PVA, polyvinyl alcohol; RPE, retinal pigment epithelial.
Moreover, using the microgroove molding technique, microgrooved nanosheets can also be fabricated from biodegradable polymers. The microgrooved nanosheets with a thickness of 84 nm (bottom part) were prepared from poly(lactic-co-glycolic acid) (PLGA) and allowed for the alignment of cells on the microgrooved surface with a groove spacing and depth of 50 and 4 μm, respectively (Figure 4b).47, 48 Notably, a microgrooved nanosheet was useful for engineering a densely packed cellular organization compared with that observed for the micropatterned nanosheet because the cells localized in the grooves as well as in the trenched portions of the ridges. Therefore, soft lithography is beneficial for the functionalization of biodegradable nanosheets, which may be applicable in tissue engineering.
Micropatterned nanosheets for local cell delivery systems
The realization of the potential of regenerative medicine is important for the effective treatment of intractable diseases. To this end, the development of bioengineering and biomaterials is expected to be effective for the delivery or transplantation of autologous and harvested cells. In addition, stem cell technology, such as the application of mesenchymal stem cells, embryonic stem cells and induced pluripotent stem cells, is actively used in the study of therapeutic resources.
Age-related macular degeneration is the major cause of visual impairment in the elderly population. The main complication is rooted in neovascularization of subretinal choroidal blood vessels, resulting in the degeneration of the retinal pigment epithelial (RPE) monolayer.49 Therefore, subretinal transplantation of RPE cells is important for regenerating retinal tissues damaged by age-related macular degeneration.50 However, the narrow subretinal space is extremely limited for the localization of transplanted cells. Therefore, the development of a local cell delivery system is required for the treatment of age-related macular degeneration. We have focused on the fabrication of micropatterned nanosheets, which are injectable through a clinical syringe into the subretinal space with RPE cells (Figure 4c).51 Micropatterned nanosheets were fabricated using a combination of spin-coating and microcontact printing. Briefly, a PLGA solution mixed with magnetic nanoparticles (for visualizing nanosheets) was spin-coated on a PDMS stamp (columnar convex portions with diameters ranging from 300 to 1000 μm). Then, the PDMS was covered with a poly(lactic-co-glycolic acid)/magnetic nanoparticles layer and stamped on a PVA-pre-coated glass substrate. The transferred PLGA/magnetic nanoparticles surface was further coated with collagen, on which the RPE cells were seeded. Finally, dissolution of the PVA layer in phosphate-buffered saline allowed the release of the free-standing micropatterned nanosheets with RPE cells.
The thickness and micropatterning size of the nanosheets, which are important parameters for injection of the cells through clinical needles, were adjusted by changing the spin-coating speed, polymer concentration and design of the PDMS stamp, respectively. The thickness-dependent flexibility allows for the shape of the nanosheet seeded with cells to change, though the size of the nanosheet exceeds the inner diameter of the needle. For example, a 170-nm-thick nanosheet with a diameter of 1000 μm was aspirated and injected through an intravenous catheter (24 G, 470-μm inner diameter) with RPE cells on the surface. Despite the mechanical stress during aspiration and injection, the RPE cells retained a monolayer structure on the nanosheet without any distortion, and the cell viability was more than 80%. Furthermore, the micropatterned nanosheet was injected into the subretinal space of a swine ocular globe, where the nanosheet was successfully released, spread and fixed to the subretinal macula. We believe that the application of injectable nanosheets for regenerative medicine is a promising technology that can improve the cell transplantation process in a minimally invasive way.
Functional polymer nanosheets for health-care monitoring
Conductive polymer nanosheets for detecting bioelectrical signals
The development of wearable electronics is expected to lighten the burden of medical examination that is imposed on patients by automatically measuring bioelectrical signals (for example, electrical activity of muscles and neural potential) in daily life.52, 53 Because of demands for miniaturization of device systems to be imperceptible to the patients, much attention has been focused on the engineering of electrical components using organic materials, such as conductive polymers.54 In this regard, we have focused on the fabrication of conductive polymer nanosheets consisting of poly(3,4-ethylenedioxythiophene)/poly(styrenesulfonate) (PEDOT:PSS) as ultrathin conductors as well as ultra-conformable electrodes for advanced wearable electronics (Figure 5a).55
(a) Skin-contact electrodes consisting of PEDOT:PSS and PDLLA nanosheets for the measurement of biological signals. (b) Measurement of sEMG through conductive nanosheets via a gripping force. Partially reproduced from ref. 55. NS, nanosheet; PEDOT:PSS, poly(3,4-ethylenedioxythiophene)/poly(styrenesulfonate); sEMG, surface electromyogram. A full color version of this figure is available at Polymer Journal online.
Conductive polymer nanosheets were designed with two layers (that is, PEDOT:PSS and PLA), in which the PLA layer served as a mechanical support for the PEDOT:PSS layer. PLA was coated on a roll of polypropylene film with a Microgravure coater using a roll-to-roll process, and PEDOT:PSS was subsequently coated on the PLA layer as a conductive layer. Owing to the weak physical bonding between polypropylene and PLA, PLA/PEDOT:PSS nanosheets with a thickness of 240 nm (each polymer layer: 120 nm) were detached from the polypropylene film using adhesive tapes in the shape of an open square frame. Importantly, the presence of butylene glycol as a dopant in the PEDOT:PSS solution increased the conductivity to approximately 500 S cm−1. As a practical application, the free-standing conductive nanosheet was transferred to a human forearm and showed conformal contact deep into the wrinkle of the skin. Then, a surface electromyogram was measured using conductive nanosheets for recording the electric activity of skeletal muscle. The conductive nanosheets recorded an surface electromyogram derived from the gripping force of hands with a signal-to-noise ratio that was similar to that of commercial skin-adhesive electrodes, such as gel electrodes (Figure 5b). In addition, the conductive nanosheet was stable on the skin surface even in the presence of sweat. Therefore, the conductive nanosheet may be a useful electrode for analyzing bioelectrical signals during physiological movement, such as sporting activity. On the basis of the roll-to-roll fabrication of conductive nanosheets, integration of printing technology with nanosheet technology will advance the fabrication process of ultrathin wearable electronics by allowing large-scale and cost-effective fabrication.
Conclusions and Outlook
In this review, we summarized the recent advancements in nanosheet technology related to fabrication processes, physical properties and practical applications. On the basis of their adhesive and mechanical properties, free-standing polymer nanosheets exhibit outstanding properties compared with those of conventional polymer thin films. In particular, adhesive and flexible properties have led to the development of nano-adhesive plasters for surgical applications. The in vivo repair of tissue defects using nanosheets is a minimally invasive treatment that does not induce a significant inflammatory response or postsurgical tissue adhesion. Furthermore, the ultrathin structure of the nanosheets is similar to the dimensional features of basement membranes, and this structure has been exploited for the development of tissue engineering scaffolds. The microfabrication techniques can be used to tailor the surface functionality of the nanosheet, which directs cellular organization and allows for the engineering of hierarchical tissue structures. We also demonstrated the fabrication of conductive polymer nanosheets for the application of ultra-conformable electrodes for monitoring bioelectrical signals. These ultrathin electronics will be useful for integrating electronics into biological tissues, which will be the next generation of bio-hybrid systems, such as implantable biodevices, to directly connect electronics/robotics to our living bodies. We believe that nanosheet technology will contribute to the development of innovative nano-bio-electronics for future medical applications.
References
Whitesides, G. M., Mathias, J. P. & Seto, C. T. Molecular self-assembly and nanochemistry: a chemical strategy for the synthesis of nanostructures. Science 254, 1312–1319 (1991).
Förster, S. & Plantenberg, T. From self-organizing polymers to nanohybrid and biomaterials. Angew. Chem. Int. Ed. 41, 688–714 (2002).
Ringsdorf, H., Schlarb, B. & Venzmer, J. Molecular architecture and function of polymeric oriented systems: models for the study of organization, surface recognition, and dynamics of biomembranes. Angew. Chem. Int. Ed. 27, 113–158 (1988).
Kunitake, T. Ultrathin films as biomimetic membranes. Polym. J. 23, 613–618 (1991).
Costa, R. R. & Mano, J. F. Polyelectrolyte multilayered assemblies in biomedical technologies. Chem. Soc. Rev. 43, 3453–3479 (2014).
Pérez-Madrigal, M. M., Armelin, E., Puiggalí, J. & Alemán, C. Insulating and semiconducting polymeric free-standing nanomembranes with biomedical applications. J. Mater. Chem. B 3, 5904–5932 (2015).
Fujie, T., Okamura, Y. & Takeoka, S. in Functional Polymer Films (eds Knoll, W. & Advincula, R. C.) Ch. 29, 907–931 (Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany, 2011).
Fujie, T. & Takeoka, S. in Nanobiotechnology (eds Phoenix, D. A. & Waqar, A.) Ch. 6, 68–94 (One Central Press, UK, 2014).
Mamedov, A. A. & Kotov., N. A. Free-standing layer-by-layer assembled films of magnetite nanoparticles. Langmuir 16, 5530–5533 (2000).
Forrest, J. A., Dalnoki-Veress, K., Stevens, J. R. & Dutcher, J. R. Effect of free surfaces on the glass transition temperature of thin polymer films. Phys. Rev. Lett. 77, 2002–2005 (1996).
Jiang, C. & Tsukruk, V. V. Freestanding nanostructures via Layer-by-Layer assembly. Adv. Mater. 18, 829–840 (2006).
Endo, H., Kado, Y., Mitsuishi, M. & Miyashita, T. Fabrication of free-standing hybrid nanosheets organized with polymer Langmuir−Blodgett films and gold nanoparticles. Macromolecules 39, 5559–5563 (2006).
Vendamme, R., Onoue, S., Nakao, A. & Kunitake, T. Robust free-standing nanomembranes of organic/inorganic interpenetrating networks. Nat. Mater. 5, 494–501 (2006).
Liao, I., Wan, A. C. A., Yim, E. K. F. & Leong, K. W. Controlled release from fibers of polyelectrolyte complexes. J. Control. Release 104, 347–358 (2005).
Kumar, M. N., Muzzarelli, R. A., Muzzarelli, C., Sashiwa, H. & Domb, A. J. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 104, 6017–6084 (2004).
Baba, S., Midorikawa, T. & Nakano, T. Unambiguous detection of the adhesive failure of metal films in the microscratch test by waveform analysis of the friction signal. Appl. Surf. Sci. 144, 344–349 (1999).
Vlassak, J. J. & Nix, W. D. A new bulge test technique for the determination of Young's modulus and Poisson's ratio of thin films. J. Mater. Res. 7, 3242–3249 (1992).
Markutsya, S., Jiang, C., Pikus, Y. & Tsukruk., V. V. Freely suspended layer-by-layer nanomembranes: testing micromechanical properties. Adv. Funct. Mater. 15, 771–780 (2005).
Stafford, C. M., Harrison, C., Beers, K. L., Karim, A., Amis, E. J., Vanlandingham, M. R., Kim, H.-C., Volksen, W., Miller, R. D. & Simonyi, E. E. A buckling-based metrology for measuring the elastic moduli of polymeric thin films. Nat. Mater. 3, 545–550 (2004).
Fujie, T., Kawamoto, Y., Haniuda, H., Saito, A., Kabata, K., Honda, Y., Ohmori, E., Asahi, T. & Takeoka, S. Selective molecular permeability induced by glass transition dynamics of semi-crystalline polymer ultra-thin films. Macromolecules 46, 395–402 (2013).
Eling, B., Gogolewski, S. & Pennings, A. J. Biodegradable materials of poly(l-lactic acid): 1. Melt-spun and solution-spun fibres. Polymer 23, 1587–1593 (1982).
Porte, H. L., Jany, T., Akkad, R., Conti, M., Gillet, P. A., Guidat, A. & Wurtz, A. J. Randomized controlled trial of a synthetic sealant for preventing alveolar air leaks after lobectomy. Ann. Thorac. Surg. 71, 1618–1622 (2001).
Kawamura, M., Gika, M., Izumi, Y., Horinouchi, H., Shinya, N., Mukai, M. & Kobayashi, K. The sealing effect of fibrin glue against alveolar air leakage evaluated up to 48h; comparison between different methods of application. Eur. J. Cardiothorac. Surg. 28, 39–42 (2005).
Gika, M., Kawamura, M., Izumi, Y. & Kobayashi, K. The short-term efficacy of fibrin glue combined with absorptive sheet material in visceral pleural defect repair. Interact. Cardiovasc. Thorac. Surg. 6, 12–15 (2007).
Fujie, T., Okamura, Y. & Takeoka, S. Ubiquitous transference of free-standing polysaccharide nanosheet in the development of a nano-adhesive plaster. Adv. Mater. 19, 3549–3553 (2007).
Fujie, T., Matsutani, N., Kinoshita, M., Okamura, Y., Saito, A. & Takeoka, S. Adhesive, flexible and robust polysaccharide nanosheet integrated for tissue-defect repair. Adv. Funct. Mater. 19, 2560–2568 (2009).
Malangoni, M. A. Contributions to the management of intraabdominal infections. Am. J. Surg. 190, 255–259 (2005).
Brook, I. Microbiology and management of abdominal infections. Dig. Dis. Sci. 53, 2585–2591 (2008).
Fujie, T., Saito, A., Kinoshita, M., Miyazaki, H., Ohtsubo, S., Saitoh, D. & Takeoka, S. Dual therapeutic action of antibiotic-loaded nanosheets for the treatment of gastrointestinal tissue defects. Biomaterials 31, 6269–6278 (2010).
Saito, A., Miyazaki, H., Fujie, T., Ohtsubo, S., Kinoshita, M., Saitoh, D. & Takeoka, S. Therapeutic efficacy of an antibiotic-loaded nanosheet in a murine burn-wound infection model. Acta Biomater. 8, 2932–2940 (2012).
Ito, K., Saito, A., Fujie, T., Nishiwaki, K., Miyazaki, H., Kinoshita, M., Saitoh, D., Ohtsubo, S. & Takeoka, S. Sustainable antimicrobial effect of silver sulfadiazine-loaded nanosheets on infection in a mouse model of partial-thickness burn injury. Acta Biomater. 24, 87–95 (2015).
Ostrovidov, S., Hosseini, V., Ahadian, S., Fujie, T., Prakash Parthiban, S., Ramalingam, M., Bae, H., Kaji, H. & Khademhosseini, A. Skeletal muscle tissue engineering: methods to form skeletal myotubes and their applications. Tissue Eng. Part B Rev. 20, 403–436 (2014).
Ghaemmaghami, A. M., Hancock, M. J., Harrington, H., Kaji, H. & Khademhosseini, A. Biomimetic tissues on a chip for drug discovery. Drug Discov. Today 17, 173–181 (2012).
Ricotti, L. & Menciassi, A. Engineering stem cells for future medicine. IEEE Trans. Biomed. Eng. 60, 727–734 (2013).
Khademhosseini, A., Langer, R., Borenstein, J. T. & Vacanti, J. P. Microscale technologies for tissue engineering and biology. Proc. Natl Acad. Sci. USA 103, 2480–2487 (2006).
Hollister, S. J. Porous scaffold design for tissue engineering. Nat. Mater. 4, 518–524 (2005).
Fu, C., Grimes, A., Long, M., Ferri, C. G. L., Rich, B. D., Ghosh, S., Ghosh, S., Lee, L. P., Gopinathan, A. & Khine, M. Tunable nanowrinkles on shape memory polymer sheets. Adv. Mater. 21, 4472–4476 (2009).
Liang, D., Hsiao, B. S. & Chu, B. Functional electrospun nanofibrous scaffolds for biomedical applications. Adv. Drug Deliv. Rev. 59, 1392–1412 (2007).
Schuurman, W., Khristov, V., Pot, M. W., van Weeren, P. R., Dhert, W. J. A. & Malda, J. Bioprinting of hybrid tissue constructs with tailorable mechanical properties. Biofabrication 3, 021001 (2011).
Tawfick, S., De Volder, M., Copic, D., Park, S.-J., Oliver, C. R., Polsen, E. S., Roberts, M. J. & Hart, A. J. Engineering of micro- and nanostructured surfaces with anisotropic geometries and properties. Adv. Mater. 24, 1628–1674 (2012).
Huh, D., Hamilton, G. A. & Ingber, D. E. From 3D cell culture to organs-on-chips. Trends Cell Biol. 21, 745–754 (2011).
Fujie, T., Ahadian, S., Liu, H., Chang, H., Ostrovidov, S., Wu, H., Bae, H., Nakajima, K., Kaji, H. & Khademhosseini, A. Engineered nanomembranes for directing cellular organization towards flexible biodevices. Nano Lett. 13, 3185–3192 (2013).
Bettinger, C. J., Langer, R. & Borenstein, J. T. Engineering substrate topography at the micro- and nanoscale to control cell function. Angew. Chem. Int. Ed. 48, 5406–5415 (2009).
Hosseini, V., Ahadian, S., Ostrovidov, S., Camci-Unal, G., Chen, S., Kaji, H., Ramalingam, M. & Khademhosseini, A. Engineered contractile skeletal muscle tissue on a microgrooved methacrylated gelatin substrate. Tissue Eng. Part A 18, 2453–2465 (2012).
Günther, A., Yasotharan, S., Vagaon, A., Lochovsky, C., Pinto, S., Yang, J., Lau, C., Voigtlaender-Bolz, J. & Bolz, S.-S. A microfluidic platform for probing small artery structure and function. Lab Chip 10, 2341–2349 (2010).
Yuan, B., Jin, Y., Sun, Y., Wang, D., Sun, J., Wang, Z., Zhang, W. & Jiang, X. A strategy for depositing different types of cells in three dimensions to mimic tubular structures in tissues. Adv. Mater. 24, 890–896 (2012).
Shi, X., Fujie, T., Saito, A., Takeoka, S., Hou, Y., Shu, Y., Chen, M., Wu, H. & Khademhosseini, A. Periosteum-mimetic structures made from freestanding microgrooved nanosheets. Adv. Mater. 26, 3290–3296 (2014).
Fujie, T., Shi, X., Ostrovidov, S., Liang, X., Nakajima, K., Chen, Y., Wu, H. & Khademhosseini, A. Spatial coordination of cell orientation directed by unique nanoribbon sheets. Biomaterials 53, 86–94 (2015).
Hynes, S. R. & Lavik, E. B. A tissue-engineered approach towards retinal repair: scaffolds for cell transplantation to the subretinal space. Graefes. Arch. Clin. Exp. Ophthalmol. 248, 763–778 (2010).
Binder, S., Stanzel, B. V., Krebs, I. & Glittenberg, C. Transplantation of the RPE in AMD. Prog. Retin. Eye Res. 26, 516–554 (2007).
Fujie, T., Mori, Y., Ito, S., Nishizawa, M., Bae, H., Nagai, N., Onami, H., Abe, T., Khademhosseini, A. & Kaji, H. Micropatterned polymeric nanosheets for local delivery of an engineered epithelial monolayer. Adv. Mater. 26, 1699–1705 (2014).
Fukuda, K., Takeda, Y., Yoshimura, Y., Shiwaku, R., Tran, L. T., Sekine, T., Mizukami, M., Kumaki, D. & Tokito, S. Fully-printed high-performance organic thin-film transistors and circuitry on one-micron-thick polymer films. Nat. Commun. 5, 4147 (2014).
Dagdeviren, C., Shi, Y., Joe, P., Ghaffari, R., Balooch, G., Usgaonkar, K., Gur, O., Tran, P. L., Crosby, J. R., Meyer, M., Su, Y., Chad Webb, R., Tedesco, A. S., Slepian, M. J., Huang, Y. & Rogers, J. A. Conformal piezoelectric systems for clinical and experimental characterization of soft tissue biomechanics. Nat. Mater. 14, 728–736 (2015).
Rivnay, J., Owens, R. M. & Malliaras, G.G. The rise of organic bioelectronics. Chem. Mater. 26, 679–685 (2014).
Zucca, A., Yamagishi, K., Fujie, T., Takeoka, S., Mattoli, V. & Greco, F. Roll to roll processing of ultraconformable conducting polymer nanosheets. J. Mater. Chem. C 3, 6539–6548 (2015).
Acknowledgements
This work was partially supported by JSPS KAKENHI (grant number 15H05355 for TF) from MEXT, Japan, the Precursory Research for Embryonic Science and Technology (PRESTO) from the Japan Science and Technology Agency (JST; grant number 15655478 for TF), and Mizuho Foundation for the Promotion of Sciences (TF). The author sincerely acknowledge Professor Shinji Takeoka and Mr Kento Yamagishi at Waseda University, Professor Daizoh Saitoh and Associate Professor Manabu Kinoshita at the National Defense Medical College, Associate Professor Yosuke Okamura at Tokai University, Professor Ali Khademhosseini at the Harvard-MIT Division of Health Sciences and Technology, Associate Professor Hirokazu Kaji and Professor Toshiaki Abe at Tohoku University, and Dr Alessandra Zucca, Dr Francesco Greco and Dr Virgilio Mattoli at the Italian Institute of Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares no conflict of interest.
Rights and permissions
About this article
Cite this article
Fujie, T. Development of free-standing polymer nanosheets for advanced medical and health-care applications. Polym J 48, 773–780 (2016). https://doi.org/10.1038/pj.2016.38
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pj.2016.38
This article is cited by
-
Enhanced cellular engraftment of adipose-derived mesenchymal stem cell spheroids by using nanosheets as scaffolds
Scientific Reports (2021)
-
Tissue-adhesive wirelessly powered optoelectronic device for metronomic photodynamic cancer therapy
Nature Biomedical Engineering (2018)
-
Cellular behaviors on polymeric scaffolds with 2D-patterned mechanical properties
Polymer Journal (2018)
-
Renewable polymeric materials for electronic applications
Polymer Journal (2017)
-
On the injectability of free-standing magnetic nanofilms
Biomedical Microdevices (2017)