Abstract
The present article reports a new method for the synthesis of ‘artificial urushi’ using cardanol, a major ingredient of cashew nutshell liquid, as the starting monomer. Natural urushi sap is polymerized in a water-in-urushiol (w/o) emulsion system; here, we mimic this system for the polymerization of cardanol. The combination of a weak amine base and a weak carboxylic acid functions as efficient emulsifier. The polymerization of cardanol is carried out in a water-in-cardanol (w/o) emulsion to produce a prepolymer emulsion, which is further cured by crosslinking, resulting in the biomimetic polymerization of cardanol. The prepolymer emulsion is stable for storage. The films were prepared from the prepolymer emulsion; the curing of these films was achieved by thermal annealing or by cobalt naphthenate-catalyzed crosslinking in air to generate ‘artificial urushi’. The rheological properties of the cardanol monomer and the prepolymer emulsions resulted in excellent film formation on the substrate. Physical property examinations of the cured films showed sufficient hardness for use as a coating layer. Cardanol is a renewable resource, and no organic solvent was used, and thus, this polymerization is a green chemical process.
Similar content being viewed by others
Introduction
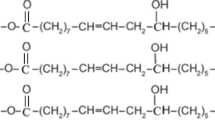
‘Urushi’ is a unique natural macromolecule, formed from polymerization and curing processes of ‘urushiol’, a substituted catechol harvested from the urushi tree (Rhus vernicifera). Urushi is the only example in nature of a polymer that is formed not only by the action of the natural laccase enzyme but also cured via oxidation in air. Urushi has been used as a traditional Japanese lacquer (more widely, oriental lacquer) since ancient times, over 9000 years ago.1, 2, 3, 4, 5, 6 Accordingly, the structure of urushiol and its hardening mechanism to form urushi have been studied by chemists for more than a century.7, 8, 9, 10, 11, 12 Urushiol is a catechol natural product with a saturated or unsaturated C15 hydrocarbon chain at the 3-position. Natural urushi is formed by the laccase-catalyzed polymerization of urushiol in air, using oxygen as an oxidant, to give a ‘prepolymer’ (first step), followed by curing via slow air oxidation of the prepolymer (second step; Scheme 1).1, 2, 3, 6
Natural urushi has long been used as a coating and adhesive material because it is hard enough to be brilliantly polished, highly durable and solvent resistant. However, the expense of urushi has limited its use, and production of natural urushi in Japan has dropped. Therefore, we have sought to create a similar material, ‘artificial urushi’1, 3, 13, 14, 15, 16, 17 (or ‘man-made urushi’2), via in vitro synthesis in a less expensive manner, with the aim of establishing practical applications. The applications do not necessarily target performance materials such as natural urushi, but rather commodity-use materials, for example, to replace natural urushi. For this purpose, urushiol analogs were investigated as starting compounds, including 4-hydroxyphenethyl alcohol derivatives containing an unsaturated fatty acid group,13, 14 catechol derivatives containing an unsaturated fatty oil group15, 16 and naphthol derivatives with a linseed oil or fish oil component.17
Cardanol, a phenol derivative with a meta-substituent of a C15 saturated or unsaturated hydrocarbon chain with one to three double bonds, is inexpensively obtained as a major component of cashew nutshell liquid, and hence attracted our attention as a possible urushiol analog.18, 19, 20 The oxidative polymerization of cardanol was performed using iron-N,N′-ethylenebis(salicylideneamine) (Fe–salen) in an organic solvent (Scheme 2).18, 20 Soybean peroxidase enzyme was also active as a catalyst for the polymerization.19 The polymerization was examined under various reaction conditions in organic solvents or in bulk.18, 20 Fe–salen (a model complex of peroxidase) was found to be more active for the cardanol oxidative polymerization compared with the Cu-containing laccase enzyme that catalyzes natural urushi formation or various transition metal complexes.18, 20, 21, 22 Interestingly, a Cu-containing tyrosinase model complex showed selective oxidative polymerization catalysis for a phenolic monomer to give poly(phenylene oxide).21, 22 For the polymerization of cardanol, however, the polymerization using an organic solvent is not desirable from a green viewpoint. In addition, when the bulk polymerization was conducted starting at room temperature, the reaction system reached 140 °C due to an exothermic reaction that could not be controlled. The addition of water to the reaction system did not suppress the temperature increase, and a partial gelation of prepolymer occurred in some cases.20
Natural urushi sap forms a water-in-oil emulsion (w/o, water-in-urushiol) to give a stable prepolymer solution via laccase-catalyzed polymerization, leading to natural urushi after air oxidation.1, 23 As an extension of our previous studies18, 19, 20, 21, 22, 23 and to improve upon the above reports, we sought to mimic the emulsion system of natural urushi. Thus, we have explored a new biomimetic oxidative polymerization of cardanol, which is carried out with using a w/o (water-in-cardanol) emulsion system for the synthetic approach to artificial urushi. This study provides not only a novel biomimetic reaction like that of natural urushi but also a green reaction system using a renewable biomass, cardanol, as the monomer without an organic solvent. The use of the stable homogeneous prepolymer emulsion solution leads to a smooth film formation as well as a cured hard film formation. The polymerization of cardanol and other compounds from cashew nutshell liquid have been under continuous study.24, 25, 26, 27, 28 Cardanol and polycardanol have been used for materials applications and modifications.29, 30, 31, 32 Natural urushiol and urushi have recently been characterized as thermosetting polymers.32, 33, 34 In many cases, these polymers are derived from renewable resources and obtained using enzymatic catalysis, which renders them excellent examples of ‘green polymer chemistry’.35, 36, 37, 38, 39, 40
Experimental procedures
Materials
Cardanol (purity: 95.4%, containing 4.6% cardol) was kindly supplied by Tohoku Chemical Industries, Ltd., Nasu-uyama City, Tochigi, Japan. Poly(ɛ-lysine) (viscous liquid from soybean) was supplied by JNC Co., Tokyo, Japan. Chitosan (oligomer) was obtained from Koyo Chem. Co., Ltd, Chiyoda-ku, Tokyo, Japan. Polyethyleneimines of molecular weight 600, 1800 and 10 000 (PEI600, 1800 and 10 000, respectively), diethylamine, triethylamine and Fe–salen were purchased from Tokyo Kasei Chem. Co., Tokyo, Japan and were used as received. Sodium dodecylbenzene sulfonate, sodium oleate, gelatin (fine powder), poly(vinyl alcohol) (degree of polymerization=1000), 30% hydrogen peroxide (H2O2), cobalt naphthenate and acetic acid (AA) were purchased from Nakalai Tesque Co., Kyoto, Japan and used as received. Lecithin (from soybean) was obtained from Kanto Kagaku, Co., Tokyo, Japan. Hexaglyceryl condensed ricinoleic acid was obtained from Taiyo Chem. Co., Tokyo, Japan. Poly(ethylene glycol)99-b-poly(propylene glycol)69-b-poly(ethylene glycol)99 (PlulonicF127) was purchased from Sigma-Aldrich Japan, Co., Tokyo, Japan.
Formation of water-in-cardanol (w/o) emulsion
In a typical reaction, an emulsifier (6.0 mg; 1.0 wt% cardanol) was added to a mixture of water (0.60 g) and cardanol (0.60 g, 2.0 mmol), with stirring for several minutes at room temperature. To obtain an emulsion, an additional emulsifier AA (molar unit equivalent amount to emulsifier) was added to the mixture with stirring.
Oxidative polymerization of cardanol
A typical run (code 2, Table 2) was as follows. To an emulsion prepared from water (0.60 g), cardanol (0.60 g, 2.0 mmol), PEI1800 (6.0 mg, 1.0 wt% with respect to cardanol), AA (8.4 mg, equimolar to the amino unit amount) and Fe–salen (4.0 mg, 0.012 mmol) in a flask, was added 30% H2O2 (80 μl, 0.71 mmol) dropwise 10 times under stirring for 2 h, keeping the flask at 20 °C in a water bath. The reaction mixture was further stirred for 24 h at the same temperature. A viscous oily prepolymer product was obtained.
Curing of poly(cardanol)
Curing of the prepolymers was carried out in two ways. (1) The cardanol prepolymer film was prepared on a glass plate using an applicator. The film was annealed at 150 °C in air for 1 h and further at room temperature for 1 day and 10 days. (2) Cobalt naphthenate (5 wt% with respect to the prepolymer)-catalyzed crosslinking reaction of the prepolymer film was carried out in air at room temperature for 3, 5 and 10 days. The thickness of the films ranged from 35 to 45 μm, depending on the emulsion water content.
Analytical methods
Size exclusion chromatographic analysis was carried out using a Shimadzu LC-20AD with a Shimadzu RID-10A RI (refractive index, Kyoto, Japan) detector and a Shimadzu CTO-10A column heater. A Tosoh TSkgel SuperHZM-N column was used with chloroform eluent. Polystyrene standards were used to obtain molecular weight calibration curves; the value limit was in the range of 270 to 700 000 Da. Optical microscopy was conducted by using a digital microscope (VHX-200: Keyence, Osaka, Japan). The rheological properties of cardanol and the prepolymer emulsions were determined at 25 °C with a parallel plate rheometer (Bohlin Gemini HR nano: Malvern, Worcestershire, UK). The diameter of the plates was 25 mm, and the gap between the plates was set to 0.5 mm. Thermo-mechanical analysis of the cured films was carried out with a dynamic mechanical analyzer (Rheogel-E4000: UBM, Kyoto, Japan) in a tensile mode. Rectangular samples of 16 mm × 5 mm in size were cut from the cured films and set between a pair of clamps 6 mm apart. The heating rate and the frequency were set at 3 °C min−1 and 16 Hz, respectively. The hardness of the cured film was evaluated by the pencil scratch method by using Mitsubishi pencil Hi-Uni 6B–6H, according to JIS K-5400.
Results and Discussion
The cardanol polymerization is outlined in Scheme 2, involving polymerization and curing steps. The polymerization uses a Fe–salen complex as catalyst, as it was found to be the most active catalyst for forming the prepolymer (Scheme 3).20
Natural urushi sap is known to polymerize in a water-in-oil (w/o) emulsion system to produce a urushiol prepolymer solution, eventually giving rise to natural urushi upon exposure to air; the sap is composed of urushiol (55–70%), water-soluble polysaccharides (6–10%), glycoproteins (1–3%), the laccase enzyme (<1%) and water (20–30%).23, 40, 41, 42, 43, 44 It is believed that the glycoproteins act as an emulsifier, leading to excellent natural urushi.
The search for an emulsifier to form water-in-cardanol (w/o) emulsions
To mimic the natural urushi system, a w/o (water-in-cardanol) emulsion must be created. To find the appropriate emulsifier, screening of known emulsifiers was conducted in a mixture of water/cardanol (50/50 weight ratio). The emulsion formation was evaluated as to whether a solution, milk-like in appearance, was formed. The ratio is important (vide infra)—here, a 50/50 ratio was used—and the amount of emulsifier used was 1.0 wt% with respect to cardanol (Table 1). Surfactants can act as emulsifiers, so various surfactants have been examined as potential candidates. Well-known surfactants did not give good results (codes 1–3). The phospholipid lecithin (code 4) and polymeric surfactants such as gelatin, chitosan, poly(vinyl alcohol) and PluronicF127 were not effective on their own (codes 5–8). As the phenolic OH of cardanol is slightly acidic, a weak amine base was examined, expecting that an acid–base interaction may be effective. To the mixture of water and cardanol, a high or a low molecular weight amine was added with stirring; however, no emulsion formation was observed with the amine alone (codes 9–14).
Acetic acid (AA, equimolar amount for the amino-group units) was added to the mixture of water–cardanol–PEI (polyethyleneimine) with stirring, clearly giving rise to an emulsified solution (codes 22, 24 and 26). A water–cardanol–poly(ɛ-lysine) mixture also behaved similarly upon the addition of AA (code 27). Although the addition of 2.0 wt% base emulsifier with AA was notably successful (code 25), 0.5 wt% did not perform as well (code 23), likely due to insufficient emulsifier. The combination of other surfactants or low molecular weight amines with AA did not display effective emulsifier behavior (codes 15–21, 28 and 29). Thus, the emulsifier PEI1800-AA was used mostly in further experiments.
The emulsion was significantly more stable on addition of AA to an amino-group-containing oligomer or polymer emulsifier (codes 22 and 24–27). The function of AA is speculated in Figure 1. Without AA, the phenolic OH groups interact with the amino groups very weakly, causing coalescence to form large water droplets (left). On addition of AA, ammonium ions are formed, causing repulsion that stabilizes the small water droplets (right). In addition to effecting repulsion, multiple ammoniums on the same molecule (for example, poly(ɛ-lysine)) form a micron-sized water particle; a small molecule such as diethylamine, would only serve to increase the number of ammoniums cations.
Other acids besides AA may work similarly as an emulsifying agent. In fact, preliminary observations showed that acrylic acid functioned such as AA. To our knowledge, this result represents the first example of the combined use of a weak base (oligomeric or polymeric) and a weak acid for use as an effective emulsifying system.
Direct observation of w/o emulsions
The water/cardanol weight ratio (%) was varied from 50/50, 33/67, 20/80 to 10/90, and a milk-like solution was observed in all cases. Emulsion formation was verified by direct observation of the mixture using optical microscopy. In these four mixtures, cardanol (1.2 g=4.0 mmol), PEI1800 (12 mg, 1.0 wt% with respect to cardanol=0.28 mmol amine equiv.), and AA (17 mg=0.28 mmol) were contained and the amount of water was varied. Figure 2 shows the optical micrographs of the four mixtures. In solution (Figure 2a), which had a 50/50 ratio, water droplets of the size less than approximately 20 μm were clearly visible in the condensed state. In the dark part of the image, water is spread and smaller water droplets are formed. White, cloudy regions represent the oily cardanol.
As the water proportion decreased, that is, in Figures 2b–d, similar water droplets were observed, with diameters less approximately 15 μm, 15 μm and 10 μm, respectively. Dark and white cloudy regions were common for Figures 2b–d. These observations confirm that these four mixtures form w/o emulsions. A similar 67/33 mixture (wt%) did not give an emulsion, but a phase-separated mixture was observed, suggesting that to obtain a stable w/o emulsion system, the proportion of water should be less than that of cardanol. The w/o emulsion of natural urushi sap exhibits a similar optical micrograph, as previously described.41
Polymerization of cardanol to a prepolymer emulsion and its film formation property
On the basis of the above observations, the oxidative polymerization was carried out using the 33/67 wt% water/cardanol emulsion system using Fe–salen exclusively as the polymerization catalyst. Figure 3 shows optical micrographs of the four steps toward the polymerization. Figure 3a indicates no emulsion formation from the mixture, as seen in code 10 (Table 1). The mixture is composed of water droplets of diameter between approximately 130 μm and 5 μm; phase separation occurred within half a day. On the addition of AA to the mixture in Figure 3a, emulsion formation was observed, as shown in Figure 3b (see code 24, Table 1). The addition of the Fe–salen catalyst (4.0 mg) with stirring did not affect the emulsion, as shown in Figure 3c. Fe–salen was likely present in the cardanol phase, as it is more soluble in cardanol than in water. The polymerization was effected by the addition of the oxidant, 30 wt% H2O2, with stirring. After the polymerization, a milk-like emulsion solution of the prepolymer was obtained, as shown in Figure 3d. Figure 3d clearly indicates that the reaction system resulted an emulsion similar to the biomimetic polymerization reaction discussed above.
The prepolymer emulsion solution can be stably stored at room temperature for longer than 1 month when air is removed from the solution. Similar stability is observed in natural urushi solutions.
Table 2 shows the results of the oxidative polymerization of cardanol conducted under various conditions and the film formation properties of the prepolymer emulsions. In all polymerization runs, the monomer conversion was in between 47% and 58%, with Mn values of 5.6–7.0 × 103. Prepolymer emulsion systems were observed in all polymerizations, except for codes 5 and 6, which also resulted in coated films for the measurement of hardness. Poly(ɛ-lysine) also functioned like PEI as an efficient emulsifier component (code 8).
Five prepolymers were prepared via oxidative polymerization, varying the feed water/cardanol ratio and subjected to film formation. The film was obtained using an applicator on the glass slide. Figure 4 shows the appearances of the films before (Figure 4a) and after (Figure 4b) and (Figure 4c) curing. In all the polymerization reactions, 69 μl water was theoretically liberated from 80 μl 30 wt% H2O2. The liberated water neither disturbed the polymerization nor the emulsion solution. In all the cases, water condensed on the glass flask surface as droplets during the polymerization. For the film formation using an applicator, the prepolymer emulsions with higher water content, such as 50/50, 33/67 and 20/80, proceeded smoothly to yield the film, whereas the bulk reaction and the emulsions with a lower water content, that is, 10/90, did not result in film formation, as seen in Figure 4a.
The prepolymer emulsions from the ratios of 50/50, 33/67 and 20/80 proceeded smoothly to afford the cured films, as seen in (b) and (c); the films from 10/90 and the bulk reaction, however, did not give such cured films as stated above. The bulk polymerization behavior (see also code 6 in Table 2) is slightly different from that reported in a previous study, in which prepolymer obtained in bulk successfully resulted in film formation.20 The difference may be owing to the purity of the starting cardanol: approximately 95% in this work and 84% in the previous work (vide infra).
Rheological properties of water/cardanol and water/prepolymer emulsions
The shear rate dependence on the steady shear viscosity of the cardanol monomer and its emulsions are shown in Figure 5. The bulk cardanol monomer shows a Newtonian viscosity of 0.04 Pa s, much lower than that of pure urushiol (approximately 0.5 Pa s), as previously reported.41 Although the molecular weights of the cardanol monomer and urushiol are nearly identical, approximately 300, the stronger acidity of the urushiol, a catechol derivative, results in a stronger tendency to form hydrogen bonded molecular clusters, which increases its viscosity. Figure 5 clearly indicates that the increase of the water content in the water/cardanol emulsions resulted not only in an increase in the steady shear viscosity at low shear rates but also in the appearance of strong non-Newtonian effects, that is, a shear rate dependence on the steady shear viscosity.
The steady shear viscosities of the water/prepolymer emulsions with various water content are shown in Figure 6. The water/prepolymer emulsions exhibited strong shear thinning character that is similar to what was observed in the water/cardanol emulsions. The shear thinning characteristic tends to be stronger with increasing water content, and the viscosity of the emulsions approached that of pure prepolymer at higher shear rates. The absolute values of the dynamic viscosity and the storage and loss moduli of the water/prepolymer emulsions are shown in Figure 7. The tendency of the dynamic viscosity, shown in Figure 8, is very similar to that of the steady shear viscosity. A strong shear thinning behavior was observed for emulsions with 20 to 50 wt% of water, but the bulk prepolymer and the emulsion with 10 wt% of water exhibited Newtonian behavior. Similarly, emulsions with 20 to 50% of water showed strong elastic behavior, while the pure prepolymer and the emulsion with 10 wt% of water showed liquid-like behavior.
As observed in Figures 6, 7 and 8, the rheological properties clearly explain the differences in the applicability of the prepolymer emulsions onto the glass plate. The prepolymer emulsions were spread onto the glass plate using an applicator with a 0.5 mm gap at approximately 10 cm s−1 (Figure 4), which corresponds to a shear rate of 2 × 102 s−1. The viscosity of the prepolymer emulsions decreases to that of the bulk prepolymer at this shear rate, and the emulsions are well spread into a thin film on the substrate. Once the shear stress is released, the liquid film regains its emulsion structure instantaneously, resulting in a significant increase in the viscosity, nearing that of the original emulsion, which suppresses the repulsion between the cardanol prepolymer film and the glass plate. This peculiar rheological property makes the cardanol prepolymer emulsion suitable as a coating material on the substrate. The fine water droplets dispersed in the emulsions may increase its overall hydrophilicity, which would assist with the smooth spreading of the emulsions on the glass plate.
Physical properties of cured films
The hardness of the cured films is listed in Table 3. Thermally cured films showed hardness of 3H and 4H at 1 and 10 days after curing, indicating that the hardness was maximized after 1 day. The hardness of the film chemically cured with the cobalt naphthenate catalyst was 2H after 3 days and 3H after 5 and 10 days with PEI+AA (codes 2–4), whereas the hardness was slightly lower with poly(ɛ-lysine)+AA (code 8). A hardness value of 2H–4H is tough enough for practical applications.20 Moreover, these films appear smooth and brilliant.
The storage modulus E′ and the loss tangent tan δ of the thermally and chemically cured films are plotted in Figures 9a and b, respectively, as functions of temperature. Thermally cured films of the two prepolymer emulsions in Figure 9a showed E′ ranging from 0.6 to 0.7 GPa at room temperature, and tan δ peaked at around 75 °C. The bulk prepolymer was prepared with or without the addition of PEI+AA, as in the preparation of the prepolymer emulsions. The cured film of the bulk prepolymer showed E′ of 0.45 GPa and a peak of tan δ at 68 °C with PEI+AA and 0.5 GPa and 72 °C without PEI+AA. These results indicate that the PEI mildly plasticized the cured film. The E′ gradually decreased to 15–20 MPa with increasing temperature and remained at those values at a higher temperature range. The E′ and tan δ of the films cured by the addition of cobalt naphthenate are shown in Figure 9b. These films showed a higher E′, although the temperature dependence is very similar to that of the thermally cured films. The tan δ of the chemically cured films also showed similar temperature dependence to that of the thermally cured films. The tan δ peak temperature of the film from the prepolymer emulsion (water/cardanol=33/67) was 65 °C, and that of the bulk prepolymer without PEI+AA was 72 °C. It is interesting to note that the film of prepolymer emulsified by poly(ɛ-lysine)+AA showed a slightly higher E′ and a higher tan δ peak temperature of approximately 75 °C.
Thermo-mechanical analyses of thermally and chemically cured films of cashew nutshell liquid polymerized in 1,4-dioxane as well as conventionally cured natural urushi were performed in our previous study.20 The CNLS used in that previous study was industrial grade and contained 83–84% cardanol, 8–11% cardol and 2% 2-methylcardol, all of which are catechol derivatives, while the cashew nutshell liquid used in this study contains 95% cardanol and 5% cardol. CNLS films cured by cobalt naphthenate in the previous study showed a slightly higher E′ and a lower tan δ peak temperature of approximately 60 °C, although their temperature dependences were very similar to those observed in this study. However, the thermally cured CNLS film and the natural urushi film cured by the conventional process did not show clear tan δ peaks, and their E′ decreased with temperature to a less significant degree than those of the thermally cured film examined here. These differences suggest an effect from the cardol and 2-methylcardol content, which would result in crosslinking structures different from those in the films from the cardanol prepolymer with lower cardol content.
Conclusion
A biomimetic reaction was developed to identify polymerization conditions for a water-in-oil (w/o) emulsion formation to obtain ‘artificial urushi’ from cardanol. Combination of an oligo- or polyamine and acetic acid (AA) served as an excellent emulsifier. Fe–salen catalyzed polymerization of cardanol in the emulsion proceeded smoothly to produce a prepolymer emulsion, which resulted in good films for curing. The prepolymer emulsion resulted in better film formation compared to the bulk prepolymer. This prepolymer emulsion can be stored for longer than 1 month in the absence of air and can then be used to form a coated film. The biomimetic polymerization solves nearly all the problems associated with non-emulsive polymerization. The cured film exhibited strong hardness, as evaluated by the hardness test and thermo-mechanical analysis, and it appeared smooth and brilliant, similar to natural urushi. Thus, the present emulsion system provides a new coating material for potentially practical applications in commodity use. Furthermore, this study used a renewable bio-based cardanol and no organic solvent, resulting in a good example of green polymer chemistry.

Structure of urushiol and the two-step reaction leading to natural urushi.

Structure of cardanol and reaction processes leading to artificial urushi.

Structure of Fe–salen complex.
References
Kobayashi, S., Uyama, H. & Ikeda, R. Artificial urushi. Chem. Eur. J. 7, 4755–4760 (2001).
Ikeda, R., Tsujimoto, T., Tanaka, H., Oyabu, H., Uyama, H. & Kobayashi, S. Man-made urushi-preparation of crosslinked polymeric films from renewable resources via air-oxidation processes. Proc. Jpn Acad., Ser. B 76, 155–160 (2000).
Kobayashi, S. Artificial urushi. J. Adhesion Soc. Jpn 49, 468–473 (2013).
Rogue, B. V. A. History of Japanese Lacquer Work, (University of Toronto Press, Toronto, Ontario, 1976).
Vogl, O. Oriental lacquer, poison ivy, and drying oils. J. Polym. Sci. Part A Polym. Chem. 38, 4327–4335 (2000).
Kumanodani, J. in Polymeric Materials Encyclopedia (ed. Salamone J. C.) 4835–4842 (CRC Press, Inc., Boca Raton, FL, 1996).
Yoshida, H. Chemistry of lacquer (Urushi). Part I. Communication from the Chemical Society of Tokio. J. Chem. Soc., Trans. 43, 472–486 (1883).
Bertrand, G. Sur le latex de l'abre laque. Compt. Rend. 118, 1213–1218 (1894).
Majima, R. Über den Hauptbestandteil des Japanlacks. (I. Mitteilung.) Über urushiol und urushioldimethyläthe. Ber. Dtsch. Chem. Ges. B 42, 1418–1423 (1909).
Majima, R. Über den Hauptbestandteil des Japan-Lacks, IX. Mitteilung: chemische Untersuchung der verschiedenen natürlichen Lackarten, die dem Japan-Lack nahe verwandt sind. Ber. Dtsch. Chem. Ges. B 55, 191–214 (1922).
Symes, W. F. & Dawson, C. R. Poison ivy “urushiol”. J. Am. Chem. Soc. 76, 2959–2963 (1954).
Loev, B. & Dawson, C. R. On the geometrical configuration of the olefinic components of poison ivy urushiol. The synthesis of a model compound. J. Am. Chem. Soc. 78, 1180–1183 (1956).
Tsujimoto, T., Ikeda, R., Uyama, H. & Kobayashi, S. Synthesis and curing of crosslinkable polyphenols from urushiol analogues. Chem. Lett. 29, 1122–1123 (2000).
Tsujimoto, T., Ikeda, R., Uyama, H. & Kobayashi, S. Crosslinkable polyphenols from urushiol analogues. Macromol. Chem. Phys. 202, 3420–3425 (2001).
Kobayashi, S., Ikeda, R., Oyabu, H., Tanaka, H. & Uyama, H. Artificial urushi: design, synthesis, and enzymatic curing of new urushiol analogues. Chem. Lett. 29, 1214–1215 (2000).
Ikeda, R., Oyabu, H., Tanaka, H., Uyama, H. & Kobayashi, S. Preparation of artificial urushi via an environmentally benign process. Bull. Chem. Soc. Jpn. 74, 1067–1073 (2001).
Tsujimoto, T., Uyama, H. & Kobayashi, S. Synthesis and curing behaviors of cross-linkable polynaphthols from renewable resources: preparation of artificial urushi. Macromolecules 37, 1777–1782 (2004).
Ikeda, R., Tanaka, H., Uyama, H. & Kobayashi, S. A new crosslinkable polyphenol from a renewable resource. Macromol. Rapid Commun. 21, 496–499 (2000).
Ikeda, R., Tanaka, H., Uyama, H. & Kobayashi, S. Enzymatic synthesis and curing of poly(cardanol). Polym. J. 32, 589–593 (2000).
Ikeda, R., Tanaka, H., Uyama, H. & Kobayashi, S. Synthesis and curing behaviors of a crosslinkable polymer from cashew nut shell liquid. Polymer 43, 3475–3481 (2002).
Higashimura, H., Kubota, H., Shiga, A., Fujisawa, K., Moro-oka, Y., Uyama, H. & Kobayashi, S. “Radical-controlled” oxidative polymerization of 4-phenoxyphenol by a tyrosinase model complex catalyst to poly(1,4-phenylene oxide). Macromolecules 33, 1986–1995 (2000).
Kobayashi, S. & Higashimura, H. Oxidative polymerization of phenols revisited. Prog. Polym. Sci. 28, 1015–1048 (2003).
Kobayashi, S. Chemistry Today 28–35 (Tokyo Kagaku Dojin, Tokyo, 2003).
Kim, Y. H., An, E. S., Song, B. K. & Kim, D. S. Polymerization of cardanol using soybean peroxidase and its potential application as anti-biofilm material. Biotechnol. Lett. 25, 1521–1524 (2003).
Won, K., Kim, Y. H., An, E. S., Lee, Y. S. & Song, B. K. Horseradish peroxidase-catalyzed polymerization of cardanol in the presence of redoxm mediators. Biomacromolecules 5, 1–4 (2004).
Tsujimoto, T., Ando, N., Oyabu, H., Uyama, H. & Kobayashi, S. Laccase-catalyzed curing of natural phenolic lipids and product properties. J. Macromol. Sci. Pure Appl. Chem A44, 1055–1060 (2007).
Park, S. Y., Kim, Y. H., Won, K. & Song, B. K. Enzymatic synthesis and curing of polycardol from renewable resources. J. Mol. Catal. B Enzym. 57, 312–316 (2009).
Chelikani, R., Kim, Y., Yoon, D.-Y. & Kim, D.-S. Enzymatic polymerization of natural anacardic acid and antibiofouling effects of polyanacardic acid coatings. Appl. Biochem. Biotechnol. 157, 263–277 (2009).
Souza, F. G., Richa, P., de Siervo, A., Oliveira, G. E., Rodrigues, D. H. M., Nele, M. & Pinto, J. C. New in situ blends of polyaniline and cardanol bio-resins. Macromol. Mater. Eng. 293, 675–683 (2008).
Shibata, M., Itakura, Y. & Watanabe, H. Bio-based thermosetting resins composed of cardanol novolac and bismaleimide. Polym. J. 45, 758–765 (2013).
Zhou, Q., Cho, D. W., Song, B. K. & Kim, H. J. Curing behavior of polycardanol by MEKP and cobalt naphthenate using differential scanning calorimetry. J. Therm. Anal. Calorim. 99, 277–284 (2010).
Voirin, C., Caillol, S., Sadavarte, N. V., Tawade, B. V., Boutevin, B. & Wadgaonkar, P. P. Functionalization of cardanol: towards biobased polymers and additives. Polym. Chem. 5, 3142–3162 (2014).
Watanabe, H., Fujimoto, A. & Takahara, A. Characterization of catechol-containing natural thermosetting polymer “urushiol” thin film. J. Polym. Sci. Part A Polym. Chem. 51, 3688–3692 (2013).
Watanabe, H., Fujimoto, A. & Takahara, A. Surface texturing of natural ‘urushi’ thermosetting polymer thin films. Polym. J. 46, 216–219 (2014).
Yamaguchi, S., Tanha, M., Hult, A., Okuda, T., Ohara, H. & Kobayashi, S. Green polymer chemistry: lipase-catalyzed synthesis of bio-based reactive polyesters employing itaconic anhydride as a renewable monomer. Polym. J. 46, 2–13 (2014).
Yoshimura, Y., Nojiri, M., Arimoto, M., Ishimoto, K., Aso, Y., Ohara, H., Yamane, H. & Kobayashi, S. Green polymer chemistry: one-pot, metal-free synthesis of macromonomer via direct polycondensation of lactic acid and its radical polymerization to graft and comb polymers. Polymer 90, 342–350 (2016).
Kobayashi, S., Uyama, H. & Kimura, S. Enzymatic polymerization. Chem. Rev. 101, 3793–3818 (2001).
Kobayashi, S. & Makino, A. Enzymatic polymer synthesis: an opportunity for green polymer chemistry. Chem. Rev. 109, 5288–5353 (2009).
Kobayashi, S. Green polymer chemistry: recent developments. Adv. Polym. Sci. 262, 141–166 (2013).
Shoda, S., Uyama, H., Kadokawa, J., Kimura, S. & Kobayashi, S. Enzymes as green catalyst for precision macromolecular synthesis. Chem. Rev. 116, 2307–2413 (2016).
Amari, T., Kumanotani, J. & Achiwa, M. Viscoelastic properties of a sap of Japanese lac trees and the lac from it. J. Jpn. Soc. Color Mater. 53, 629–634 (1980).
Matsui, E. Urushi kagaku (Urushi chemistry), (Nikkan Kogyo Sinbun Co., Tokyo, 1997).
Miyakoshi, T., Nagase, K. & Yoshida, T. Urushi kagaku no simpo (Developments of urushi chemistry), (IPC Co., Tokyo, 2000).
Obataya, E., Furuta, Y., Ohno, Y., Norimoto, M. & Tomita, B. Effect of aging and moisture on the dynamic viscoelastic properties of oriental lacquer (urushi) film. J. Appl. Polym. Sci. 83, 2288–2294 (2002).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Otsuka, T., Fujikawa, Si., Yamane, H. et al. Green polymer chemistry: the biomimetic oxidative polymerization of cardanol for a synthetic approach to ‘artificial urushi’. Polym J 49, 335–343 (2017). https://doi.org/10.1038/pj.2016.118
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pj.2016.118
This article is cited by
-
Nature-inspired materials: Emerging trends and prospects
NPG Asia Materials (2021)
-
Preparation of biobased wrinkled surfaces via lignification-mimetic reactions and drying: a new approach for developing surface wrinkling
Polymer Journal (2017)
-
Green polymer chemistry: new methods of polymer synthesis using renewable starting materials
Structural Chemistry (2017)