Abstract
In this study we developed hydrogel-based hybrid materials with gold nanoparticles that have tunable optical properties under light irradiation. Attaining the stimuli-responsive dynamic changes in the solid material from the optical properties of the gold nanoparticles depended on the interparticle distance, and we designed hydrogel-based organic–inorganic hybrids with gold nanoparticles. Homogeneously dispersed states of the modified gold nanoparticles were achieved in the hydrogel with the interaction of the imidazolium units tethered to both the nanoparticle surfaces and the side chains of the networks. Initially, we found that the absorption properties could be changed by swelling and shrinking the hydrogels. Next, by using the photo-responsive mono-carboxylate linker, we induced the photo-triggered modulation of the absorption properties of the hydrogels. These results can be explained by a change in the interparticle distance within the hydrogel matrix.
Similar content being viewed by others
Introduction
The development of organic–inorganic hybrid materials is a topic of great importance because of the high potential of these materials to present unique properties different from the intrinsic characteristics of each component. In particular, because of their high transparency and durability, hybrid materials are regarded as a suitable scaffold for the construction of functional optical materials.1,2,3 Recently, using hybridization to fix unstable nanostructures or short-lived states inside materials has allowed the achievement of a number of interesting properties, such as oxygen-resistant phosphorescence4 and multi-colored emissions.5, 6 Moreover, by employing heteroatom-containing functional units called as the element blocks,7 the introduction of new functionalities was accomplished, including the ability to generate heat under microwave irradiation.8, 9 Furthermore, the disadvantages of conventional hybrids, such as low conductivity, were overcome.10 In general, owing to the intrinsic stability of inorganic materials, hybrid materials are highly robust.11,12,13,14,15 Therefore, hybridization is a valid strategy to enhance the thermal and mechanical properties of polymeric materials.16 However, this also means that the hybrids show poor tunability after shape formation. Therefore, there are still significant difficulties in preparing hybrids that demonstrate dynamic changes in their properties when exposed to external stimuli.17
Gold nanoparticles (gold atoms; Aun, n<100) exhibit a characteristic light absorption that originates from the coherent oscillation of the conduction band electrons (surface plasmon oscillations) induced by interaction with an electromagnetic field.18, 19 Because it is possible to tune their optical properties through the assembly states of the nanoparticles, biosensors or stimuli-responsive materials have been fabricated based on modified gold nanoparticles.20, 21 We have recently reported the synthesis and unique aggregation behavior of imidazolium-presenting gold nanoparticles.22, 23 The aggregation states can be observed by performing an anion exchange under mild conditions.22 In addition, the interparticle distances between the gold nanoparticles in the aggregates varied depending on the counteranion used. Moreover, water-dispersive aggregates were achieved by including a photo-responsive linker, and the interparticle distances in the aggregates were tuned by the light irradiation.23 Although we have established the simple tools needed to precisely control the aggregation morphology of the particles, these dynamic changes can be observed only in the fluid state. It is important to understand the dynamic changes in the dispersion/aggregation states of the nanoparticles under mobility-restricted conditions and to obtain the optical properties as observed in the fluids to fabricate functional materials based on the tunable properties of gold nanoparticles.
We have presented a series of organic–inorganic hybrid gels composed of organic linkers and inorganic crosslinking points.24,25,26,27 Moreover, various types of nanoparticle-based functional materials have been reported via surface modification with nanoparticles.28 To maintain the mobility of the particles and the material properties of a solid, we focused on hydrogels29,30,31 as scaffolds to achieve the stimuli-responsive dynamic changes in the solid materials, exploiting the optical properties of the gold nanoparticles. Herein, we present the preparation of hydrogel-based hybrid materials with gold nanoparticles and their tunable dynamic optical properties under light irradiation. Hydrogels with homogeneously dispersed modified gold nanoparticles were prepared by the interaction between the imidazolium units that were tethered to both the nanoparticle surface and the side chains of the network. Initially, changes in the optical properties upon swelling and deswelling the hydrogels were investigated. Next, by using a photo-responsive mono-carboxylate linker,23 regulation of the absorption properties of the hydrogels under light irradiation was performed. We found that the interparticle distance can be changed in the hydrogel matrix. These results imply that the structural restriction of the gold nanoparticles can be ignored in our hydrogel matrices, leading to the observed changes in the optical properties of the materials.
Experimental Procedure
General
All of the reactions were conducted under a nitrogen atmosphere unless otherwise stated. Chromatographic purifications were performed using Wakogel C-200 (Wako Pure Chemical Industries, Osaka, Japan). An ultrafilter unit (USY-5, molecular weight cutoff=50 000) was purchased from ADVANTEC (Tokyo, Japan). 1H and 13C nuclear magnetic resonance spectra were obtained with a JEOL EX-400 spectrometer (400 MHz) using chloroform-d1, deuterium oxide and dimethylsulfoxide-d6 as solvents and either tetramethylsilane or trimethylsilylpropionic acid sodium salt as an internal reference (JEOL, Tokyo, Japan). Ultraviolet (UV)–visible spectra were measured on a Shimadzu UV-3600 spectrophotometer using quartz cuvettes with a 1-cm optical path length (Shimadzu, Kyoto, Japan). Thermogravimetric analysis was performed using a TG/DTA6200 (Seiko Instruments Inc., Chiba, Japan) with a heating rate of 10 °C min−1 up to 900 °C under air. Fourier transform infrared spectra were recorded on a Perkin Elmer 1600 infrared spectrophotometer (PerkinElmer Inc., Waltham, MA, USA) using KBr discs dispersed with the powder samples. The samples were irradiated with UV light using a spiral-shaped, low-pressure mercury lamp. The samples were added to a quartz glass tube and placed on the center of the spiral-shaped lamp. Compound 132, 33, the photo-responsive carboxylate linker 223 and the imidazolium-presenting gold nanoparticles NPIm22 were prepared according to previous reports (Supplementary Scheme S1).
Preparation of NPIm-containing hydrogels
The typical procedure followed to prepare NPIm-containing hydrogels is described here. A mixture containing 100 mg of 1, 10 ml of an aqueous dispersion containing NPIm (1 mg, 8 × 10−4 mol of imidazolium), tetraethylene glycol diacrylate (5 mol% to 1) and VA-080 (5 mol% to 1) in the presence or absence of 2 ml of a 0.04-M aqueous solution of 2 was placed at 80 °C for 12 h in a vacuum oven. After the reaction, hydrogels with a blue to black color (depending on the ratio of NPIm/1) were obtained. FTIR: 839, 983 (–C=C–H out-of-plane bending), 1415 (–C=C–H bending) and 1630 cm−1 (–C=C– imidazolium ring stretching). Elemental analysis: calcd. for (C13H24ClN2O2)0.87(C14H22O7)0.04(C10H21ClN2S)0.02(Au)0.10(H2O)0.08: C 52.5, H 8.0 Cl 11.4, N 9.0; found: C 54.3, H 8.2 Cl 11.4, N 9.0. 1H nuclear magnetic resonance (dimethyl sulfoxide-d6, 400 MHz) δ p.p.m.: 1.24–1.37 (4H, m, –CH2CH2–CC–OCO–), 1.59 (2H, q, J=7.1 Hz, –CH2–C–OCO–), 1.75 (2H, q, J=7.1 Hz, –C3H3N2+–CH2CH2–), 3.41 (3H, s, H3C–C3H3N2+–), 4.01–4.26 (4H, m, –C3H3N2+–CH2–, –CH2–OCO–), 5.93 (1H, d, J=10.2 Hz, trans-CH2=CHCOO–), 6.15 (1H, dd, J=17.3 Hz, 10.2 Hz, geminal-CH2=CHCOO–), 6.30 (1H, d, J=17.3 Hz, cis-CH2=CHCOO–), 7.71 (1H, s, C(5)–H of imidazolium), 7.78 (1H, s, C(4)–H of imidazolium), 9.19 (1H, s, C(2)–H of imidazolium).
Determination of the swelling ratio of the hydrogels
The synthesized hydrogel (26.8 mg) with 1 wt% NPIm was soaked in 20 ml of distilled water and incubated at room temperature for 12 h. Then, the hydrogel was removed from the water and excess moisture was removed with a Kimwipe before the weight was measured (185 mg).
Photoreactions
The typical procedure is described here: the sample was placed in a quartz glass test tube that was located on the center of spiral-shaped mercury lamp. Then, the sample was irradiated with UV light at room temperature.
Results and Discussion
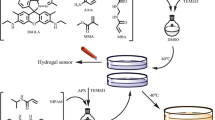
To achieve the stimuli-responsive behavior and tunable properties of the hydrogel by performing an anion exchange, we designed a hydrogel possessing imidazolium units along the side chains of the hydrogel network and the nanoparticle surface. Gold nanoparticles modified with methylimidazolium cations NPIm and vinyl monomer 1 tethering the imidazolium unit were synthesized as described in previous reports.22, 32, 33 After the preparation of each material, NPIm was dispersed in an aqueous solution of 1, and hydrogelation was induced in the presence of a water-soluble azo-initiator VA-080 and the crosslinker tetraethylene glycol diacrylate (Scheme 1). After incubation for 20 h at 80 °C, a blue-colored transparent film was obtained (Figure 1). To evaluate the maximum capacity of the hydrogel with NPIm, the polymerization was performed at various feed ratios of 1 and NPIm. After hydrogelation, the synthesized hydrogels were soaked in water (Supplementary Figure S1). Desorption was observed to occur from the hydrogel prepared using a mixture containing 100 mg of 1 and 10 mg of NPIm. Therefore, we prepared hydrogels using 1 mg of NPIm and <100 mg of 1. The products exhibited a high moisture absorbency. In addition, in the swollen state, the gels showed poor mechanical robustness. Therefore, the samples readily adopted irregular shapes, as shown in Figure 1.
The polymerization was monitored by Fourier transform infrared spectroscopy (Supplementary Figure S2). Absorptions at 1415 (out-of-plane vibration), 983 and 839 cm−1 (out-of-plane bending vibration of vinyl C-H) disappeared after a 20-h incubation period. From these results, we concluded that the polymerization was complete. From thermogravimetric analysis, the residue was 0.75 wt% (Supplementary Figure S3). This value is consistent with the theoretical weight percentage of Au as calculated from the feed ratio (0.80 wt%). These results suggest that the gold nanoparticles are incorporated quantitatively into the hydrogel networks. Elemental analysis also supports the quantitative introduction of gold nanoparticles into the hydrogel networks (calcd. (C13H24ClN2O2)0.95(C14H22O7)0.05(C10H21ClN2S)0.02(Au)0.11, found (C13H24ClN2O2)0.87(C14H22O7)0.04(C10H21ClN2S)0.02(Au)0.10(H2O)0.08).
The swelling behavior of the synthesized hydrogels was also examined (Figure 1). The synthesized hydrogels were observed to efficiently absorb water. The weight of the hydrogel was significantly elevated after swelling in distilled water to 185 from 27 mg. The swelling ratio of the hydrogel was calculated to be 691%. The hydrogel exhibited color changes before and after swelling. By washing the hydrogel with water, the color changed from blue to red. From the UV–vis absorption spectra, the absorption band at 561 nm derived from the surface plasmon resonance of the gold nanoparticles shifted to 515 nm after the washing treatment (Figure 2). These data are representative of the interparticle separation between adjacent nanoparticles within the hydrogel and indicate that the interparticle distance should be enlarged in the swollen state. It is likely that each particle is isolated by swelling the hydrogel because the nanoparticles interact strongly with the hydrogel network via the imidazolium moieties.
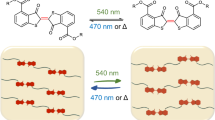
Finally, we investigated stimuli-responsive changes in the optical properties based on the gold nanoparticle-containing hydrogels. A schematic illustration of the system is shown in Scheme 2. To achieve the light-responsive behavior, we used a photo-cleavable linker.23 In the presence of the mono-carboxylate linker 2 with a photo-cleavable ester, NPIm can form water-dispersive aggregates. Triggered by UV irradiation, the linker should be transformed into the dicarboxylate, leading to tight binding with the gold nanoparticles. As a result, the observed red-shift of the absorption band can be expected, particularly when these changes proceed in the aggregates. Therefore, it may be presumed that the restricted mobility of the nanoparticles is negligible. To probe the validity of this scenario, the hydrogel was prepared using the same method described previously in the presence of 2. The gelation proceeded with the aggregation of gold nanoparticles. After washing with distilled water, red-colored hydrogels were obtained (Figure 3).
To investigate the photo-responsive behavior of these hydrogels, a low-pressure mercury lamp was used to directly irradiate the prepared hydrogels for 10 min. Before and after photo-irradiation, the UV–vis spectra were recorded (Figure 4). In the absence of 2, no significant changes were observed by UV–vis spectroscopy. In contrast, a small increase in the absorption within the longer wavelength region was observed for the hydrogel involving 2 after UV irradiation. A corresponding slight color change was observed for the sample containing 2 (Figure 3). These data indicate that the interparticle distance is reduced by UV irradiation. It should be noted that the nanoparticle aggregation can be induced by light irradiation even in the spatially restricted matrix, leading to a change in optical properties.
Conclusion
We accomplished a light-driven absorption change derived from the modulation of the interparticle distance in the aggregates of gold nanoparticles within a hydrogel matrix. Our results resolve a significant issue, that is, whether the functions originating from nanostructures can be preserved in solid materials. The detected optical properties of the hydrogel matrix originated from the dispersion and aggregation states of the gold nanoparticles. Furthermore, these changes can be induced by light irradiation. In particular, even under mobility-restricted conditions, properties derived from the aggregation state of the gold nanoparticles can be achieved. This strategy is feasible for dynamically regulating the nanostructures within the matrix. Thus, it can be said that our concept is valid for developing nanostructured materials with tunable properties.

Synthesis of the gold nanoparticle-containing hydrogels.

Schematic model of the photoreaction with the synthesized hydrogels.
References
Ohshita, J., Nakamura, M., Yamamoto, K., Watase, S. & Matsukawa, K. Synthesis of dithienogermole-containing oligo- and polysilsesquioxanes as luminescent materials. Dalton Trans. 44, 8214–8220 (2015).
Watase, S., Fujisaki, D., Watanabe, M., Mitamura, K., Nishioka, N. & Matsukawa, K. Preparation and electric property of polysilsesquioxane thin films incorporating carbazole groups. Chem. Eur. J. 20, 12773–12776 (2014).
Narisawa, M., Watase, S., Matsukawa, K., Kawai, T., Kawamoto, Y., Matsui, T. & Iwase, A. Influence of high-temperature oxidation on photoluminescent properties of white SiOC(-H) ceramics. J. Non-Cryst. Solids 391, 1–5 (2014).
Okada, H., Tanaka, K. & Chujo, Y. Regulation of responsiveness of phosphorescence toward dissolved oxygen concentration by modulating polymer contents in organic−inorganic hybrid materials. Bioorg. Med. Chem. 22, 3141–3145 (2014).
Kajiwara, Y., Nagai, A., Tanaka, K. & Chujo, Y. Efficient simultaneous emission from RGB-emitting organoboron dyes incorporated into organic-inorganic hybrids and preparation of white light-emitting materials. J. Mater. Chem. C 1, 4437–4444 (2013).
Kajiwara, Y., Tanaka, K. & Chujo, Y. Enhancement of dye dispersibility in silica hybrids through local heating induced by the imidazolium group under microwave irradiation. Polym. J. 46, 195–199 (2014).
Chujo, Y. & Tanaka, K. New polymeric materials based on element-blocks. Bull. Chem. Soc. Jpn 88, 633–643 (2015).
Okada, H., Tanaka, K. & Chujo, Y. Microwave-driven enzyme deactivation using imidazolium salt-presenting silica nanoparticles. Bioorg. Med. Chem. Lett. 24, 4622–4625 (2014).
Okada, H., Kajiwara, Y., Tanaka, K. & Chujo, Y. Rapid heat generation under microwave irradiation by imidazolium-presenting silica nanoparticles. Colloids Surf. A 428, 65–69 (2013).
Okada, H., Tanaka, K. & Chujo, Y. Preparation of environmentally resistant conductive silica-based polymer hybrids containing tetrathiafulvalen-tetracyanoquinodimethane charge-transfer complexes. Polym. J. 46, 800–805 (2014).
Tanaka, K., Adachi, S. & Chujo, Y. Structure-property relationship of octa-substituted POSS in thermal and mechanical reinforcements of conventional polymers. J. Polym. Sci. A Polym. Chem. 47, 5690–5697 (2009).
Jeon, J.-H., Tanaka, K. & Chujo, Y. POSS fillers for modulating thermal properties of ionic liquids. RSC Adv. 3, 2422–2427 (2013).
Jeon, J.-H., Tanaka, K. & Chujo, Y. Rational design of POSS fillers for simultaneous improvements of thermomechanical properties and lowering refractive indices of polymer films. J. Polym. Sci. A Polym. Chem. 51, 3583–3589 (2013).
Jeon, J.-H., Tanaka, K. & Chujo, Y. Synthesis of sulfonic acid-containing POSS and its filler effects for enhancing thermal stabilities and lowering melting temperatures of ionic liquids. J. Mater. Chem. A 2, 624–630 (2014).
Tanaka, K., Ishiguro, F., Jeon, J.-H., Hiraoka, T. & Chujo, Y. POSS ionic liquid crystals. NPG Asian Mater. 7, e174. doi:10.1038/am.2015.28 (2015).
Tanaka, K., Yamane, H., Mitamura, K., Watase, S., Matsukawa, K. & Chujo, Y. Transformation of sulfur to organic−inorganic hybrids employed by POSS networks and their application for the modulation of refractive indices. J. Polym. Sci. A Polym. Chem. 52, 2588–2595 (2014).
Okada, H., Tanaka, K., Ohashi, W. & Chujo, Y. Photo-triggered molecular release based on auto-degradable polymer-containing organic−inorganic hybrids. Bioorg. Med. Chem. 22, 3435–3440 (2014).
Daniel, M. C. & Astruc, D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 104, 293–346 (2004).
Ghosh, S. K. & Pal, T. Interparticle coupling effect on the surface plasmon resonance of gold nanoparticles: From theory to applications. Chem. Rev. 107, 4797–4862 (2007).
Saha, K., Agasti, S. S., Kim, C., Li, X. & Rotello, V. M. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 112, 2379–2779 (2012).
Sapsford, K. E., Algar, W. R., Berti, L., Gemmill, K. B., Casey, B. J., Oh, E., Stewart, M. H. & Medintz, I. L. Functionalizing nanoparticles with biological molecules: developing chemistries that facilitate nanotechnology. Chem. Rev. 113, 1904–2074 (2013).
Miyoshi, E., Naka, K., Tanaka, K., Narita, A. & Chujo, Y. Preparation of clusters having various interparticle distances based on imidazolium-modified gold nanoparticles via anion exchange. Colloids Surf. A 390, 126–133 (2011).
Tanaka, K., Naka, K., Miyoshi, E., Narita, A. & Chujo, Y. Control of interparticle spacing in stable aggregates of gold nanoparticles by light irradiation. Polym. J. (e-pub ahead of print 5 August 2015; doi:10.1038/pj.2015.56).
Jeon, J.-H., Kakuta, T., Tanaka, K. & Chujo, Y. Facile design of organic–inorganic hybrid gels for molecular recognition of nucleoside triphosphates. Bioorg. Med. Chem. Lett. 25, 2050–2055 (2015).
Jeon, J.-H., Tanaka, K. & Chujo, Y. Light-driven artificial enzymes for selective oxidation of guanosine triphosphate using water-soluble POSS network polymers. Org. Biomol. Chem. 12, 6500–6506 (2014).
Tanaka, K., Ohashi, W., Kitamura, N. & Chujo, Y. Reductive glutathione-responsive molecular release using water-soluble POSS network polymers. Bull. Chem. Soc. Jpn 84, 612–616 (2011).
Tanaka, K., Inafuku, K., Adachi, S. & Chujo, Y. Tuning of properties of POSS-condensed water-soluble network polymers by modulating the crosslinking ratio between POSS. Macromolecules 42, 3489–3492 (2009).
Tanaka, K. & Chujo, Y. Design of functionalized nanoparticles for the applications in nanobiotechnology. Adv. Powder Technol. 25, 101–113 (2014).
Ikeda, M., Tanida, T., Yoshii, T., Kurotani, K., Onogi, S., Urayama, K. & Hamachi, I. Installing logic-gate responses to a variety of biological substances in supramolecular hydrogel-enzyme hybrids. Nat. Chem. 6, 511–518 (2014).
Ikeda, M., Ochi, R., Kurita, Y., Pochan, D. J. & Hamachi, I. Heat-induced morphological transformation of supramolecular nanostructures by retro-Diels-Alder reaction. Chem. Eur. J. 18, 13091–13096 (2012).
Ikeda, M., Yoshii, T., Matsui, T., Tanida, T., Komatsu, H. & Hamachi, I. Montmorillonite-supramolecular hydrogel hybrid for fluorocolorimetric sensing of polyamines. J. Am. Chem. Soc. 133, 1670–1673 (2011).
Yoshizawa, M. & Ohno, H. Synthesis of molten salt-type polymer brush and effect of brush structure on the ionic conductivity. Electrochim. Acta 46, 1723–1728 (2001).
Washiro, S., Yoshizawa, M., Nakajima, H. & Ohno, H. Highly ion conductive flexible films composed of network polymers based on polymerizable ionic liquids. Polymer 45, 1577–1582 (2004).
Acknowledgements
This work was partially supported by a Grant-in-Aid for Scientific Research on Innovative Areas ‘New Polymeric Materials Based on Element-Blocks (No. 2401)’ (25102521) of The Ministry of Education, Culture, Sports, Science and Technology, Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on Polymer Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Tanaka, K., Naka, K., Miyoshi, E. et al. Preparation of photo-responsive hybrid materials based on hydrogels involving imidazolium-presenting gold nanoparticles. Polym J 48, 177–181 (2016). https://doi.org/10.1038/pj.2015.89
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pj.2015.89