Abstract
Polymeric biomaterials have significant impact in the aged society. Biocompatible and biodegradable polymers have emerged during the past decades to promise extraordinary breakthroughs in a wide range of diagnostic and therapeutic medical devices. Understanding and controlling the interfacial interactions of the polymeric biomaterials with biological elements, such as water, ions, proteins, bacteria, fungai and cells, are essential toward their successful implementation in biomedical applications. Here we highlight the recent developments of biocompatible and biodegradable fusion polymeric biomaterials for medical devices and provide an overview of the recent progress of the design of the multi-functional biomedical polymers by controlling bio-interfacial water structure through precision polymer synthesis and supramolecular chemistry.
Similar content being viewed by others
Introduction
In biomedical applications, there are continuous efforts to enhance methods, materials and devices. The recent development of novel biomaterials and their applications to biomedical problems have dramatically improved the treatment of many diseases and injuries.1, 2, 3 Although a various types of materials in biomedicine have been used widely, most biomaterials lack the desired functional properties to interface with biological systems and have not been engineered for optimum performance. Therefore there is an increasing demand to develop novel materials to address such problems in biomedicine arena. Biocompatible and biodegradable fusion polymers are a class of new generation of biomaterials that have demonstrated great potential for medical devices, tissue engineering scaffolds, drug delivery and biomedical-healthcare sensors.
There are numerous parameters of polymeric biomaterials that can affect the cellular behavior in a controlled manner. The underlying mechanisms for the biocompatibility of polymers at the molecular level are complex and have not been clearly demonstrated, although many theoretical and experimental efforts have been made to understand these mechanisms.4, 5 Water and proteins interactions have been recognized as fundamental for the biological response upon contact with polymers. We have proposed the ‘Intermediate Water’ concept6, 7, 8 on the basis of results on the water sorption process into polymeric biomaterials. The water exhibited clearly defined peaks for cold crystallization in the differential scanning calorimetry (DSC) chart, a strong peak at 3400 cm−1 in a time-resolved infrared spectrum and higher mobility of water in a 2H-nuclear magnetic resonance.6, 7, 8 As a result, the biocompatibility of polymers was ascribed to the predominant population of intermediate water in the hydrated polymers. Intermediate water interacts with polymer chains in a intermediate way, that is, stronger than free water but weaker than tightly bound non-freezing water. We hypothesized that intermediate water, which prevents the proteins and blood cells from directly contacting the polymer surface on the polymer surface, has an important role in the biocompatibility of polymers.
In this focus review, we describe the recent design of biocompatible and biodegradable polymeric biomaterials for various applications in medical devices. Here we present various synthetic strategies for the preparation of the biomaterials, which include characteristic properties of the biocompatibility, biodegradability and anti-microbial activity of polymer-based biomaterials in a self-organization manner. In addition, we describe the applications of polymer-based biomaterials in tissue engineering and medical devices and provide an overview of the recent experimental progress of the screening of multi-functional biocompatible polymers based on bio-interfacial water structure.
Biocompatible polymeric biomaterials
Polymeric materials for the medical devices that may come in contact with human blood should have capacity to resist protein adsorption and blood cell adhesion and thus triggering the organism’s defense systems.1 Some biocompatible polymer surfaces have been developed, and they fall into the following three categories:1, 6 (a) hydrophilic surfaces, (b) surfaces with micro-phase-separated domains, and (c) biomembrane-like surfaces, including zwitterionic groups. Physicochemical properties, including wettability, surface free energy, surface charge, stiffness, topography and the presence of specific chemical functionalities, surface bound water appears to bear an instrumental role in the biological response induced by the synthetic polymers.1, 9 New-generation polymer poly(2-methoxyethyl acrylate) (PMEA) shows excellent blood compatibility and biocompatibility and has been approved for medical use by the Food and Drug Administration.6, 7, 8 For instance, PMEA-coated circuits and tubes exhibit significantly reduced blood cell activation when used in cardiopulmonary bypass and catheters for central veins of human blood vessels. It has been maintained that PMEA’s compatibility with platelets, white and red blood cells (RBCs), complement and coagulation systems has been dictated by the presence of the intermediate water.6, 7, 8
It should be noted that the word ‘biocompatibility’ is used in general as the term evaluating properties of materials that do not cause adverse effect when the materials come into contact with living organisms, such as proteins, biological cells and tissues.5 This review primarily deals with ‘biocompatibility’ of polymer materials against various biological elements in human blood flow system.
Principle of cell attachment on polymers
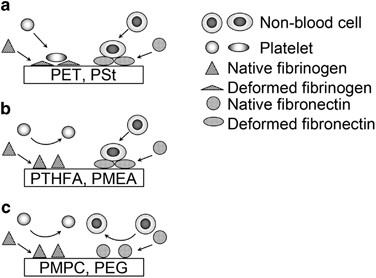
Cells can attach in serum-containing medium even on polymers, such as polystyrene and polyethylene terephthalate, which do not possess any specific cell attachment ligands.10, 11 On these polymers, serum proteins (for example, fibrinogen and fibronectin) generally adsorb and change their conformation to allow the cells to attach or to function as cell attachment ligands (Figure 1a).10, 11 Protein adsorption and its conformational change are thus critical for cell attachment on polymers, and the regulation of protein adsorption leads to the control of cell attachment on polymers. We have suggested that intermediate water can influence protein adsorption on polymers.12, 13 Therefore the attachment behavior of the cells will be different between PMEA and conventional polymers such as polystyrene due to the difference of protein adsorption and its conformational change.
Different attachment of human platelets and non-blood cells on PMEA and its analogous polymers
Cell attachment ligands are different among the cells. It has been reported that platelets require the adsorption of fibrinogen and adsorption-induced conformational change, which exposes cell attachment sites for their attachment.11 Therefore, it is necessary to prevent the adsorption and conformational change of fibrinogen on the polymers for the acquirement of blood compatibility. In contrast to platelet attachment, non-blood cells require the adsorption and the conformational change of fibronectin rather than fibrinogen for their attachment. Previously reported blood compatible polymers such as polyethylene glycol and the polymers containing 2-methacryloyloxyethyl phosphorylcholine (PMPC) have been reported to prevent the adsorption and the conformational changes of any proteins, including both fibrinogen and fibronectin, and thus any types of cells cannot attach on the substrates coated with them (Figure 1c).14, 15
PMEA and its analogous polymer, poly(tetrahydrofurfuryl acrylate) (PTHFA), have been reported as blood compatible polymers.16, 17 These polymers suppress the adsorption and conformational change of fibrinogen to prevent platelet attachment.12, 16 Recently, we have reported that PMEA and PTHFA do not suppress the conformational change of fibronectin, and the fibronectin can expose their cell attachment sites on the polymers.13 Non-blood cells can attach on PMEA and PTHFA due to such fibronectin (Figure 1b).13 PMEA and PTHFA are thus newly categorized as blood compatible polymers, which allow the attachment of non-blood cells but not platelets.13, 18
Adsorption-induced conformational change is determined by protein flexibility. The difference of conformational change between fibronectin and fibrinogen observed on PMEA and PTHFA might be due to the difference of flexibility of these proteins. Fibronectin shapes ‘beads on strings’ and shows high flexibility.19, 20 Fibronectin can change its conformation even on the polymers that prevent conformational change of adsorbed fibrinogen. Intermediate water keeps proteins away from non-freezing water, which induces conformational change of protein.12, 16 It appears that necessary amounts of intermediate water to prevent conformational change are different between fibronectin and fibrinogen due to the difference of flexibility. Therefore cell attachment can be regulated by the regulation of intermediate water content through the regulation of protein conformational change (Table 1).
Recent advances in biology and medicine require blood-contact biomedical applications, including cell isolation from blood and endothelial cell-covered artificial blood vessels and stents. Newly categorized blood-compatible polymers, such as PMEA and PTHFA, are useful for these applications. Therefore the techniques to control intermediate water contents will strongly progress blood-contact biomedical applications through the regulation of protein adsorption and the following cell attachment.
Controls on water structure at the biointerface through precision polymer synthesis
Precision control over the polymers’ biocompatibility is a longstanding drawback in the arena of biocompatible polymeric materials, and the synthesis of well-defined polymers having precisely controlled molecular architecture is a powerful approach for the manipulation of polymer properties. This is particularly true in the development of biocompatible polymeric materials where the primary structure of polymers, for example, molecular weight, molecular weight distribution, monomer sequence distribution, stereoregularity, side-chain functionality, chain-end structures and long-chain branching, can greatly affect the biocompatibility of polymeric materials. To clarify the fundamental relationship between the biocompatible property of polymers and the chemical structure of polymeric biomaterials, we have started a study to elucidate the structure-property relationships in blood-compatible polymers by means of precision polymer synthesis.
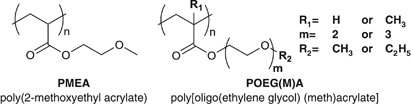
Thus far, we have been investigating the relationship between the polymer primary structures and their blood compatibility by utilizing vinyl polymers having hydrophilic functional groups. In our previous studies, we have reported that PMEA, which has a quite simple chemical structure, exhibits superior blood compatibility;21, 22, 23 and PMEA possesses the unique hydration water structure, intermediate water in the hydrated state.24, 25, 26, 27, 28, 29 We have further investigated the blood compatibility of PMEA analogous polymers (Figure 2) having differences in the backbone structure (acrylate or methacrylate), oligo(ethylene glycol) (EG) side-chain lengths (number of units=1 to 3) and side-chain terminal groups (methyl or ethyl).30
Side-chain modification
The modification with oligoEGs is a well-established methodology to tune the hydrophilicity of polymeric materials.31, 32, 33, 34, 35, 36, 37, 38, 39 Poly[oligo(ethylene glycol)(meth)acrylate]s (POEG(M)As) consist of poly(meth)acrylate backbones and oligo(ethylene glycol) side-chains, consequently, the EG functionalized poly(meth)acrylate is one of the most readily accessible hydrophilic polymers. Although POEG(M)As have simple chemical structures and numerous research studies have been conducted to date, there is still plenty of room for controlling hydrophilicity/hydrophobicity by modifying the chemical structure of side-chains. The basic way to modify the side-chain structure is by tuning the number of EG units and chain-end terminal group. Hydrophilicity of the polymer increases with the number of EG units, as the polymers have longer side-chains, the polymers become soluble in water and typically show lower critical solution temperature (LCST) in aqueous solutions.33 The number of carbon atoms in terminal alkoxy group also affects the water solubility and some of the polymers having longer alkyl terminal group show LCST below 37 °C.40 The DSC measurement revealed that intermediate water content was increased by tuning the chemical structure of polymer to be more hydrophilic (much EG units with less terminal carbons), and a decrement trend was observed in the number of adhered platelets with increasing the intermediate water content.
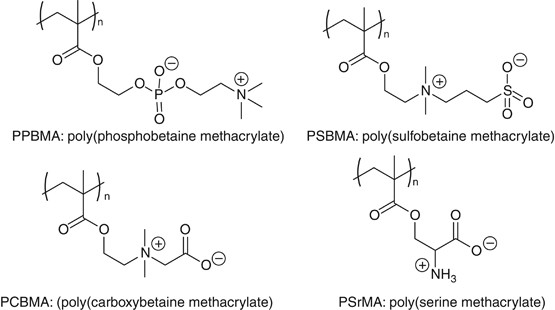
Zwitterionic polymers are known as the promising biocompatible materials for medical devices.40 For example, poly(2-methacryloyloxyethyl phosphorylcholine) (PPBMA: poly(phosphobetaine methacrylate), generally known as PMPC)) is a biomimetic material containing phosphorylcholine group for resisting nonspecific protein adsorption and platelet adhesion.41, 42 Recently, synthetic polymers containing zwitterionic structures similar to PPBMA, such as poly{[2-(methacryloyloxy)ethyl]dimethyl-(3-sulfopropyl)ammonium hydroxide} (poly(sulfobetaine methacrylate)),43, 44 and poly(1-carboxy-N,N-dimethyl-N-(2’-methacryloyloxyethyl)methanaminium) (poly(carboxybetaine methacrylate)),45, 46, 47 bearing sulfo- and carboxy- betaine group, respectively, are also reported as blood-compatible polymers, which show good plasma protein-fouling resistance. Most recently, poly(serine methacrylate) was reported as a new family of a zwitterionic polymer having an amino acid, L-serine, as the side-chain group.48 (Figure 3)
Backbone modification
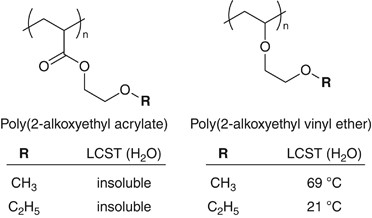
Changing the polymer backbone structure is also an effective approach to tune the polymer properties.49, 50 Poly[oligo(ethylene glycol) vinyl ether] is an analog of POEG(M)A. The structural difference between the two polymers is only in the side-chain linkage that the former OEG side-chains were connected to polymer backbone through ether bonds instead of ester connections (Figure 4). However, the difference engenders large differences in the molecular mobility and the hydrophilicity of the polymers. For instance, most of poly(vinyl ether)s having OEG side-chains show quite low glass transition temperature (Tg<−60 °C) and are soluble in water or exhibit an LCST in aqueous media. Some of the poly(vinyl ether)s (for example, poly(2-ethoxyethyl vinyl ether), LCST=21 °C) are insoluble at body temperature, and the human platelets adhesion test could be performed at 37 °C. Accordingly, we have analyzed the hydration water structure in POEG(M)A and their poly(vinyl ether) analogs by DSC, and the poly(vinyl ether)s showed a cold crystallization of water at around −40 °C and exhibited low platelet adhesion as well as the case of POEG(M)A.51
Modification on side-chain branch spacing
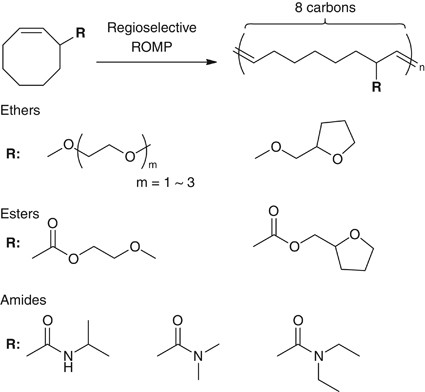
As mentioned above, the structural control over the macromolecular chemical structure is an effective approach to modify/control the hydration water structure and the blood compatibility of polymeric materials. There remains ample scope for further modification in the chemical structure of polymers, for example, tacticity, side-chain linkage and side-chain branch placement. The structural control over the side-chain placement along the polymer backbone is one of the most challenging topics in vinyl polymer synthesis. Fortunately, an effective pathway to achieve the model sequence-regulated vinyl polymers was reported, and the methodology utilizing the regio- and stereo-selective ring-opening metathesis polymerization (ROMP) of allyl-substituted cycloalkenamers52 opened a new window to precisely control the side-chain branch placement.53, 54, 55, 56, 57, 58 Based on the works, we have started a study to elucidate the structure–property relationships in biocompatible polymeric materials by means of precision polymers synthesized through regio- and stereo-selective ROMP (Figure 5).
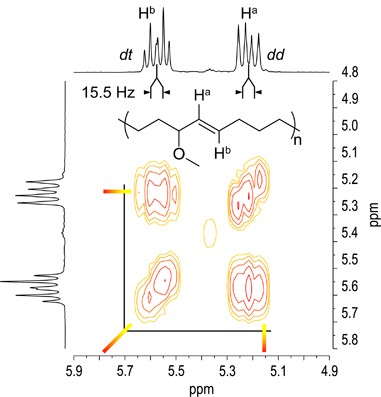
The single substitution of a functional group at the allyl-position of cis-cyclooctene (COE) allows to achieve the regioregular polymers by means of ROMP with the second-generation Grubbs catalyst (G2).59 Thus we have synthesized COEs having hydrophilic functional groups at allyl-position,60 for example, polymerized the COEs with G2 in CHCl3. ROMP of the allyl-substituted COEs proceeded in a regio- and stereo-selective manner to afford polymers exhibiting remarkably high head-to-tail regioregularity and high trans- stereo-regularity as we previously reported. Figure 6 shows olefinic region of 1H nuclear magnetic resonance and 1H-1H correlated spectra of 3-methoxy-substituted COE. The coupling constant for the two olefinic signals is Jab=15.5 Hz, indicating that the double bond has trans- configuration. The dd and dt multiplicities for Ha and Hb, respectively, and the correlation between Ha and Hb reveal the near-perfect trans- head-to-tail regularity.
Polymers having precisely placed branches on every eighth backbone carbons were obtained upon hydrogenation. Water contact angle measurement confirmed the presence of hydrophilic surface for all polymers. The water structure in hydrated polymers was determined by DSC, cold crystallization of water and/or low melting of ice in hydrated polymers were observed on heating process. Cold crystallization of water is the clear evidence for the presence of intermediate water, and the content was able to be varied by changing the polymer structure. A human platelet adhesion test was employed to assess the blood compatibility of regioselective ROMP-produced polymers. The number of adhered platelets was also varied by changing the polymer structure, and we found out that the number was suppressed by introducing the longer EG side-chains. The platelets adhesion number was decreased with increasing the content of intermediate water regardless of the polymer structure. This result suggests that our hypothesis could be true that the presence of intermediate water is the key to provide the polymer materials with antithrombotic character, and the blood compatibility of polymers should be controlled by tuning the water structure at the bio-interface through precision polymer synthesis.60
Biodegradable synthetic polymers used/studied in medical applications
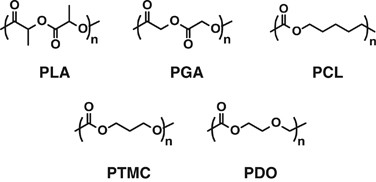
Some biomedical devices, especially for temporary use or disposable purpose, such as surgical suture, bone-fixation materials and drug-eluting stents comprise biodegradable synthetic polymers, including polylactides, polyglycolide, poly(ɛ-caprolactone), poly(trimethylene carbonate) (PTMC) and poly(p-dioxanone), as shown in Figure 7.61, 62 These polymers are degraded by hydrolysis with/without enzyme and absorbed in the body through metabolic pathway, although the duration of these existing biodegradable polymers in the body varies.63 They have drawn keen attention as alternatives to biopolymers such as peptides, nucleic acids and polysaccharides that cost high to produce and purify and potentially possess the risk of antigenicity and infection.
Such implantable medical devices need to be compatible with host cells to reduce adverse effects. As it has been confirmed that the aforementioned polymers exhibit minimal or acceptable cytotoxicity,64 most of those polymers are approved for medical application. Few reports have ever described the relationship between the biocompatibility and structural features of those polymers. In the case of PMEA, the ester and ether groups on the side-chains contribute to the hydration and generation of intermediate water.29 The hydration generally occurs through hydrogen bonding between polar moieties in the polymer and water molecules. This concept may be extended to the aforementioned polymers comprising ester or carbonate linkage and alkyl- or alkyloxy-chains of varying lengths. The detailed study for hydration and intermediate water in those polymers is now in progress by our group. Next examples also imply that the intermediate water concept should be employed to explain the observed biocompatibility.
Biodegradable antimicrobial polymers with low hemolytic property
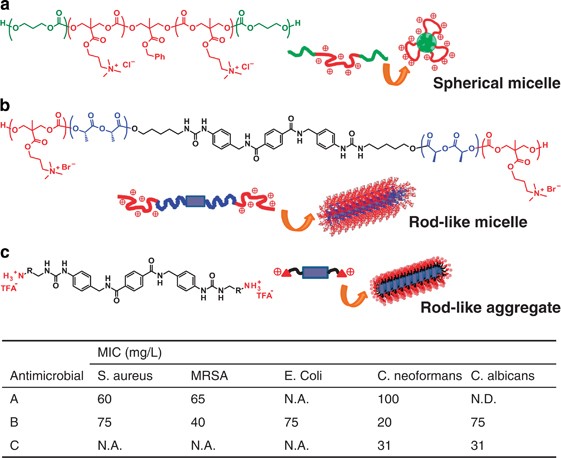
In recent years, a PTMC analog bearing a side group of quaternary ammonium salt have demonstrated potent antimicrobial activities but showed minimal hemolytic properties (Figure 8).65, 66 In contrast, most of the cationic polymers are well known to interact with negatively charged bacterial cell membranes, subsequently inducing the membrane disruption.67 As the cationic polymers physically destroy cells, drug resistance is hard to develop differently from the use of conventional antibodies. However, this electrostatic interaction often influences mammalian cells resulting in cytotoxicity, which is a serious issue to be solved in developing antimicrobial polymers with positive charges. The first antimicrobial polycarbonate reported in 2011 shows efficient antimicrobial activity but displays no hemolytic property.65 This polymer has amphiphilic triblock nature to form nano-sized micelles by conjugating hydrophobic PTMC as peripheral blocks (Figure 8a). Accumulation of positive charges on micelle surface might contribute to differentiating bacteria and mammalian cells. In similar speculation proposed by Kuroda and colleagues, localization of charges and segregation of the hydrophobic part by micellization suppress the interaction with mammalian cell membrane with less negative charges than those of bacterial cell membrane.68, 69 Considering that the other PTMC analogs bearing different side-chains have also exhibited little cytotoxicity;70, 71, 72 however, this low hemolytic property may also be supported by the contribution of hydration involving the carbonate linkages in the main chain. In particular, as both RBCs and platelets are blood cells, the inactive behavior of the polymer to RBCs is likely to occur in a similar way that PMEA shows excellent compatibility to platelets.16 IBM propounds to call a series of these antimicrobial biodegradable polycarbonates ‘Ninja Polymer’ describing the function to work behind the scenes and eventually disappear.
Supramolecularly bolstered antimicrobial activity and blood compatibility
Multiple activities against several types of bacteria present another challenge for the design of antimicrobial materials. The first antimicrobial polycarbonate described above shows the efficacy only against Gram-positive bacteria and their drug-resistant strains such as Bacillus subtilis and (methicillin-resistant) Staphylococcus aureus, respectively.65 Because Gram-negative bacteria and fungi are not as negatively charged as Gram-positive bacteria are,73 other artifices should be integrated into the macromolecular architecture. Lipophilicity and hydrophobicity are generally required for valid antimicrobial activity against Gram-negative bacteria such as Escherichia coli and Pseudomonas aeruginosa, owing to affinity to superficial lipopolysaccharide on the cell wall. However, increased hydrophobicity of the cationic polymers often develops hemolytic property. Fukushima et al.66 introduced a rigid hydrogen bonding motif in the middle of a center hydrophobic segment of a triblock copolymer composed of poly(L-lactide) (PLLA) and the cationic polycarbonate, forming fibrous micelles by orientation of self-assembly (Figure 8b). Interestingly, this polymer shows antimicrobial activity against a wide range of bacteria covering Gram-positive/negative bacteria and fungi but induced no hemolysis. In all cases, minimum inhibitory concentration of this polymer was higher than critical micelle concentration, supporting that the polymer serves as aggregates. It turns out that the fibrous shape is somewhat responsible for the improved antimicrobial activity. Later, Fukushima et al.74 have also reported supramolecular antifungals where the same rigid hydrogen bond motif is used directly to attach low molecular cationic primary ammonium at the both ends instead of the cationic polycarbonate (Figure 8c). The antifungal becomes active against fungi such as Candida albicans and Cryptococcus neoformans only in the form of nanofiber that indicates glass transition at 120 °C similar to molecular glass.75
In these above two cases, the hemolytic activity of the polymer also remained minimal. The cationic moiety obviously affects bacterial cell membranes, but the interaction with RBCs is mitigated even though the assembly form varies. At the latter case, especially, the interaction of molecules with cells, such as cytotoxicity, antimicrobial activity and biocompatibility, is managed by cooperation of primary structure of the peripheral functional groups to tune the chemical functions and higher-order structure forming specific shape to restrict or expand the chemical function as biological system generally adopts. The cationic moiety usually forms hydration layer, including strongly oriented water molecules that are categorized as non-freezing water, often causing adverse effects. If the intermediate water is responsible for the low hemolytic property of the cationic fibrous assemblies, the following hypothesis would be supposed: By assembling to such fibrous form, the surface hydration layer is disorganized with electrostatic repulsion of condensed cationic groups, which may trigger forming intermediate water from non-freezing water. In fact, a similar insight has been reported for generation of intermediate water by disorganization of hydration layer of non-freezing water in a copolymer of poly[N-methyl-N-(4-vinylphenethyl)ethylenediamine] with a small amount of additional poly(2-hydroxyethyl methacrylate).76
Control over the geometry of polymeric aggregates often entails non-covalent interactions, including hydrogen bond, π-π stacking, charge transfer complex and ion complex. These interactions also differentiate nano-rheology and dynamics of the aggregates. According to Stupp and colleagues, strength of the interaction at the internal domain of cationic supramolecular aggregates affects accumulation on and disaggregation of mammalian cell membrane.77 The aggregates with strong ‘internal bond strength’ interact with the cell membrane resulting in membrane disruption, while those with weak internal bond strength remain dynamic nature to release unimers upon approaching cells leading to no damage of the cells. It turns out that the selection of types and direction of bond (covalent vs non-covalent) significantly involves regulation of biocompatibility and cytotoxicity. In consequence, design of high-performance biomaterials in future should actively employ supramolecular chemistry in terms of geometry control, subsequent development of secondary function and dynamic behavior of the material besides primary chemical functions.
The research on clarification of relationship between biocompatibility of these polymer systems and water structure is ongoing.78 The intermediate water was only found in hydrated biopolymers (proteins, polysaccharides and nucleic acid; DNA and RNA) and hydrated biocompatible synthetic polymers but not in hydrated non-biocompatible synthetic polymers.79, 80, 81, 82 Therefore we propose intermediate water concept for directional design of functional polymeric biomaterials, but it is needed for the quantitative and precise description of biocompatibility driven by novel interface-sensitive approaches, such as spectroscopic (including sum-frequency generation and dielectric spectroscopy), X-ray and neutron scattering, and force curve measurements combined with computer simulations under the physiological condition.83, 84, 85, 86, 87, 88, 89, 90, 91, 92, 93, 94
Conclusion
Surfaces made of biocompatible and biodegradable polymers profoundly influence cell behavior at all hierarchical levels. The interaction of polymers with cells is managed by cooperation of primary structure including backbone and functional groups at the side-chain to tune the chemical functions and higher-order structure forming specific shape to restrict or expand the chemical function as biological system generally adopts. Using principles of intermediate water, which is common in hydrated biopolymers and in only biocompatible synthetic polymers, the synthetic and supramolecular methodology to create novel biocompatible polymers moves toward a more high-throughput way. Such well-defined polymeric biomaterials could find application in the age of personalized medicine.
References
Severian, D . Polymeric Biomaterials, (Mercel Dekker, New York, NY, USA, 2002).
Karagkiozaki, V . & Logothetidis, S . Horizons in Clinical Nanomedicine, (Pan Stanford Publishing Pte. Ltd., Singapore, Singapore, 2014).
Bouten, C. V. C ., Dankers, P. Y. W ., Driessen-Mol, A ., Pedron, S ., Brizard, A. M. A . & Baaijens, F. P. T . Substrates for cardiovascular tissue engineering. Adv. Drug Deliv. Rev. 63, 221–241 (2011).
Ratner, B. D . Blood compatibility-a prespective. J. Biomater. Sci. Polym. Ed. 11, 1107–1119 (2000).
Tsuruta, T . On the role of water molecules in the interface between biological systems and polymers. J. Biomater. Sci. Polym. Ed. 21, 1831–1848 (2010).
Tanaka, M ., Hayashi, T . & Morita, S . The roles of water molecule at the biointerface of medical polymers. Polym. J. 45, 701–710 (2013).
Javakhishvili, I ., Tanaka, M ., Ogura, K ., Jankova, K . & Hvilsted, S . Synthesis of graft copolymers based on poly(2-methoxyethyl acrylate) and investigation of the associated water structure. Macromol. Rapid Commun. 33, 319–325 (2012).
Miwa, Y ., Ishida, H ., Saitô, H ., Tanaka, M . & Mochizuki, A . Network structures and dynamics of dry and swollen poly(acrylate)s.Characterization of high- and low-frequency motions as revealed by suppressed or recovered intensities (SRI) analysis of 13C NMR. Polymer 50, 6091–6099 (2009).
Nell, A. E ., Mädler, L ., Velegol, D ., Xia, T ., Hoek, E. M. V ., Somasundaran, P ., Klaessig, F ., Castranova, V . & Thompson, M . Understanding biophysicochemical interactions at the nano-bio interface. Nat. Mater. 8, 543–557 (2009).
Grinnell, F . & Feld, M. K . Fibronectin adsorption on hydrophilic and hydrophobic surfaces detected by antibody binding and analyzed during cell adhesion in serum-containing medium. J. Biol. Chem. 257, 4888–4893 (1982).
Sivaraman, B . & Latour, R. A . The relationship between platelet adhesion on surfaces and the structure versus the amount of adsorbed fibrinogen. Biomaterials 31, 832–839 (2010).
Tanaka, M ., Mochizuki, A ., Shiroya, T ., Motomura, T ., Shimura, K ., Onishi, M . & Okahata, Y . Study on kinetics of earlystage protein adsorption on poly(2-methoxethylacrylate) (PMEA) surface. Coll. Surf. A Physicochem. Eng. Aspects 203, 195–204 (2002).
Hoshiba, T ., Nikaido, M . & Tanaka, M . Characterization of the attachment mechanisms of tissue-derived cell lines to blood-compatible polymers. Adv. Healthcare Mater. 3, 775–784 (2014).
Zhang, Z ., Zhang, M ., Chen, S ., Horbett, T. A ., Ratner, B. D . & Jiang, S . Blood compatibility of surfaces with superlow protein adsorption. Biomaterials 29, 4285–4291 (2008).
Ishihara, K ., Aragaki, R ., Ueda, T ., Watanabe, A . & Nakabayashi, N . Reduced thrombogenicity of polymers having phospholipid polar groups. J. Biomed. Mater. Res. 24, 1069–1077 (1990).
Tanaka, M ., Motomura, T ., Kawada, M ., Anzai, T ., Kasori, Y ., Shiroya, T ., Shimura, K ., Onishi, M . & Mochizuki, A . Blood compatible aspects of poly (2-methoxyethylacrylate) (PMEA)- relationship between protein adsorption and platelet adhesion on PMEA surface. Biomaterials 21, 1471–1481 (2000).
Mochizuki, A ., Hatakeyama, T ., Tomono, Y . & Tanaka, M . Water structure and blood compatibility of poly (tetrahydrofurfuryl acrylate). J. Biomater. Sci. Polym. Ed. 20, 591–603 (2009).
Kitakami, E ., Aoki, M ., Sato, C ., Ishihata, H . & Tanaka, M . Adhesion and proliferation of human periodontal ligament cells on poly(2-methoxyethyl acrylate). Biomed. Res. Int. 2014, 102648 (2014).
Erickson, H. A ., Carrell, N . & McDonagh, J . Fibronectin molecule visualized in electron microscopy: a long, thin, flexible strand. J. Cell Biol. 91, 673–678 (1981).
Leahy, D. J ., Aukhil, I . & Erickson, H. P . 2.0 Å Crystal structure of a four-domain segment of human fibronectin encompassing the RGD loop and synergy region. Cell 84, 155–164 (1996).
Tanaka, M . & Mochizuki, A . Clarification of the blood compatibility mechanism by controlling the water structure at the blood-poly(meth)acrylate interface. J. Biomater. Sci. Polym. Ed. 21, 1849–1863 (2010).
Tanaka, M ., Mochizuki, A ., Ishii, N ., Motomura, T . & Hatakeyama, T . Study on blood compatibility of poly(2-methoxyethylacrylate). Relationship between water structure and platelet compatibility in poly(2-methoxyethylacrylate-co-2- hydroxyethylmethacrylate). Biomacromolecules 3, 36–41 (2002).
Tanaka, M ., Mochizuki, T ., Motomura, T ., Shimura, K ., Onishi, M . & Okahata, Y . In Situ studies on protein adsorption onto a poly(2-methoxyethylacrylate) surface by a quartz crystal microbalance. Coll. Surf. A Physicochem. Eng. Aspects 193, 145–152 (2001).
Tanaka, M ., Motomura, T ., Ishii, N ., Shimura, K ., Onishi, M ., Mochizuki, A . & Hatakeyama, T . Cold crystallization of water in hydrated poly(2-methoxyethyl acrylate) (PMEA). Polym. Int. 49, 1709–1713 (2000).
Tanaka, M . & Mochizuki, A . Effect of water structure on blood compatibility, thermal analysis of water in poly(meth)acrylate. J. Biomed. Mater. Res. 68A, 684–695 (2004).
Miwa, Y ., Ishida, H ., Tanaka, M . & Mochizuki, A . H-2-NMR and C-13-NMR study of the hydration behavior of poly(2-methoxyethyl acrylate), poly(2-hydroxyethyl methacrylate) and poly(tetrahydrofurfuryl acrylate) in relation to their blood compatibility as biomaterials. J. Biomater. Sci. Polym. Ed. 21, 1911–1924 (2010).
Miwa, Y ., Tanaka, M . & Mochizuki, A . Water structure and polymer dynamics in hydrated blood compatible polymers. Kobunshi Ronbunshu 68, 133–146 (2011).
Hayashi, T ., Tanaka, M ., Yamamoto, S ., Shimomura, M . & Hara, M . Direct observation of interaction between proteins and blood-compatible polymer surfaces. Biointerphases 2, 119–125 (2007).
Morita, S ., Tanaka, M . & Ozaki, Y . Time-resolved in situ ATR-IR observations of the process of sorption of water into a poly(2-methoxyethyl acrylate) film. Langmuir 23, 3750–3761 (2007).
Tanaka, M . & Ido, N . Novel biocompatible and temperature responsive polymers. Patent application JP2011, 4853905.
Knop, K ., Hoogenboom, R ., Fischer, D . & Schubert, U. S . Poly(ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. 49, 6288–6308 (2010).
Veronese, F. M . & Pasut, G . PEGylation, successful approach to drug delivery. Drug Discov. Today 10, 1451–1458 (2005).
Lutz, J.-F . Polymerization of oligo(ethylene glycol) (meth)acrylates: Toward new generations of smart biocompatible materials. J. Polym. Sci. A Polym. Chem 46, 3459–3470 (2008).
Han, S ., Hagiwara, M . & Ishizone, T . Synthesis of thermally sensitive water-soluble polymethacrylates by living anionic polymerizations of oligo(ethylene glycol) methyl ether methacrylates. Macromolecules 36, 8312–8319 (2003).
Kitano, H ., Hirabayashi, T ., Gemmei-Ide, M . & Kyogoku, M . Effect of macrocycles on the temperature-responsiveness of poly (methoxy diethylene glycol methacrylate)-graft-PEG. Macromol. Chem. Phys. 205, 1651–1659 (2004).
Zhao, B ., Li, D. J ., Hua, F. J . & Green, D. R . Synthesis of thermosensitive water-soluble polystyrenics with pendant methoxyoligo(ethylene glycol) groups by nitroxide-mediated radical polymerization. Macromolecules 38, 9509–9517 (2005).
Neugebauer, D . Graft copolymers with poly(ethylene oxide) segments. Polym. Int. 56, 1469–1498 (2007).
Ishizone, T ., Seki, A ., Hagiwara, M ., Han, S ., Yokoyama, H ., Oyane, A ., Deffieux, A . & Carlotti, S . Anionic polymerizations of oligo(ethylene glycol) alkyl ether methacrylates: Effect of side chain length and ω-alkyl group of side chain on cloud point in water. Macromolecules 41, 2963–2967 (2008).
Yamanaka, J ., Kayasuga, T ., Ito, M ., Yokoyama, H . & Ishizone, T . Synthesis of water-soluble poly[oligo(ethylene glycol) methacrylate]s by living anionic polymerization of oligo(ethylene glycol) vinyl ether methacrylates. Polym. Chem. 2, 1837–1848 (2011).
Tarannum, N . & Singh, M . Advances in synthesis and applications of sulfo and carbo analogues of polybetaines: a review. Rev. Adv. Sci. Eng. 2, 90–111 (2013).
Ishihara, K ., Nomura, H ., Mihara, T ., Kurita, K ., Iwasaki, Y . & Nakabayashi, N . Why do phospholipid polymers reduce protein adsorption? J. Biomed. Mater. Res. 39, 323–330 (1998).
Ishihara, K ., Hanyuda, H . & Nakabayashi, N . Synthesis of phospholipid polymers having a urethane bond in the side chain as coating material on segmented polyurethane and their platelet adhesion-resistant properties. Biomaterials 16, 873–879 (1995).
Jun, Z ., Youling, Y ., Kehua, W ., Jian, S . & Sicong, L . Surface modification of segmented poly(ether urethane) by grafting sulfo ammonium zwitterionic monomer to improve hemocompatibilities. Colloids Surf. B 28, 1–9 (2003).
Lee, W.-F . & Tsai, C.-C . Synthesis and solubility of the poly(sulfobetaine)s and the corresponding cationic polymers: 1. Synthesis and characterization of sulfobetaines and the corresponding cationic monomers by nuclear magnetic resonance spectra. Polymer 35, 2210–2217 (1994).
Kitano, H ., Tada, S ., Mori, T ., Takaha, K ., Gemmei-Ide, M ., Tanaka, M ., Fukuda, M . & Yokoyama, Y . Correlation between the structure of water in the vicinity of carboxybetaine polymers and their blood-compatibility. Langmuir 21, 11932–11940 (2005).
Zhang, Z ., Chen, S . & Jiang, S . Dual-functional biomimetic materials: nonfouling poly(carboxybetaine) with active functional groups for protein immobilization. Biomacromolecules 7, 3311–3315 (2006).
Yuan, Y ., Zang, X ., Ai, F ., Zhou, J ., Shen, J . & Lin, S . Grafting sulfobetaine monomer onto silicone surface to improve haemocompatibility. Polym. Int. 53, 121–126 (2004).
Liu, Q ., Singh, A . & Liu, L . Amino acid-based zwitterionic poly(serine methacrylate) as an antifouling material. Biomacromolecules 14, 226–231 (2013).
Fernández-García, M ., Cuervo-Rodriguez, R . & Madruga, E. L . Glass transition temperature of methyl methacrylate–ethyl α-benzoyloxymethylacrylate copolymers. Polym. Int. 49, 377–381 (2000).
Cao, C . & Lin, Y . Correlation between the glass transition temperatures and repeating unit structure for high molecular weight polymers. J. Chem. Inf. Comput. Sci. 43, 643–650 (2003).
Tanaka, M . Blood compatible poly(vinyl ether)s. Patent application pending JP2014-047347.
Kobayashi, S ., Pitet, L. M . & Hillmyer, M. A . Regio- and stereoselective ring-opening metathesis polymerization of 3-substituted cyclooctenes. J. Am. Chem. Soc. 133, 5794–5797 (2011).
Jeong, H ., Kozera, D. J ., Schrock, R. R ., Smith, S. J ., Zhang, J ., Ren, N . & Hillmyer, M. A . Z-Selective ring-opening metathesis polymerization of 3-substituted cyclooctenes by monoaryloxide pyrrolide imido alkylidene (MAP) catalysts of molybdenum and tungsten. Organometallics 32, 4843–4850 (2013).
Martinez, H ., Miro, P ., Charbonneau, P ., Hillmyer, M. A . & Cramer, C. J . Selectivity in ring-opening metathesis polymerization of Z-cyclooctenes catalyzed by a second-generation Grubbs catalyst. ACS Catal 2, 2547–2556 (2012).
Martinez, H ., Ren, N ., Matta, M. E . & Hillmyer, M. A . Ring-opening metathesis polymerization of 8-membered cyclic olefins. Polym. Chem. 5, 3507–3532 (2014).
Pitet, L. M ., Zhang, J . & Hillmyer, M. A . Sequential ROMP of cyclooctenes as a route to linear polyethylene block copolymers. Dalton Trans. 42, 9079–9088 (2013).
Zhang, J ., Matta, M. E . & Hillmyer, M. A . Synthesis of sequence-specific vinyl copolymers by regioselective ROMP of multiply substituted cyclooctenes. ACS Macro Lett. 1, 1383–1387 (2012).
Zhang, J ., Matta, M. E ., Martinez, H . & Hillmyer, M. A . Precision vinyl acetate/ethylene (VAE) copolymers by ROMP of acetoxy-substituted cyclic alkenes. Macromolecules 46, 2535–2543 (2013).
Martinez, H ., Zhang, J ., Kobayashi, S ., Xu, Y ., Pitet, L. M ., Matta, M. E . & Hillmyer, M. A . Functionalized regio-regular linear polyethylenes from the ROMP of 3-substituted cyclooctenes. Appl. Pet. Res. (doi: 10.1007/s13203-014-0048-z).
Tanaka, M ., Kobayashi, S ., Fukuda, K . & Herai, K . Biocompatible polymers having precisely placed side-chain branches. Patent application pending JP2014, 105221.
Nair, L. S . & Laurencin, C. T . Biodegradable polymers as biomaterials. Prog. Polym. Sci. 32, 762–798 (2007).
Tian, H ., Tang, Z ., Zhuang, X ., Chen, X . & Jing, X . Biodegradable synthetic polymers: preparation, functionalization and biomedical application. Prog. Polym. Sci. 37, 237–280 (2012).
van der Zee, M . in: Handbook of Biodegradable Polymers: Isolation, Synthesis, Characterization and Applications (eds Lendlein A. & Sisson A.) 263–281 (Wiley-VCH, Weinheim, Germany, 2011).
Tzoneva, R ., Seifert, B ., Behl, M . & Lendlein, A . Elastic multiblock copolymers for vascular regeneration: protein adsorption and hemocompatibility. Clin. Hemorheol. Micro 52, 337–348 (2012).
Nederberg, F ., Zhang, Y ., Tan, J. P. K ., Xu, K ., Wang, H ., Yang, C ., Gao, S ., Guo, X. D ., Fukushima, K ., Li, L ., Hedrick, J. L . & Yang, Y. Y . Biodegradable nanostructures with selective lysis of microbial membranes. Nat. Chem. 3, 409–412 (2011).
Fukushima, K ., Tan, J. P. K ., Korevaar, P. A ., Yang, Y. Y ., Pitera, J ., Nelson, A ., Maune, H ., Coady, D. J ., Frommer, J. E ., Engler, A. C ., Huang, Y ., Xu, K ., Ji, Z ., Qiao, Y ., Fan, W ., Li, L ., Wiradharma, N ., Meijer, E. W . & Hedrick, J. L . Broad-spectrum antimicrobial supramolecular assemblies with distinctive size and shape. ACS Nano 6, 9191–9199 (2012).
Wimley, W. C . Describing the mechanism of antimicrobial peptide action with the interfacial activity model. ACS Chem. Biol. 5, 905–917 (2010).
Oda, Y ., Kanaoka, S ., Sato, T ., Aoshima, S . & Kuroda, K . Block versus random amphiphilic copolymers as antibacterial agents. Biomacromolecules 12, 3581–3591 (2011).
Palermo, E. F . & Kuroda, K . Structural determinants of antimicrobial activity in polymers which mimic host defense peptides. Appl. Microbiol. Biotechnol. 87, 1605–1615 (2010).
Attia, A. B. E ., Yang, C ., Tan, J. P. K ., Gao, S ., Williams, D. F ., Hedrick, J. L . & Yang, Y. Y . The effect of kinetic stability on biodistribution and anti-tumor efficacy of drug-loaded biodegradable polymeric micelles. Biomaterials 34, 3132–3140 (2013).
Nederberg, F ., Appel, E ., Tan, J. P. K ., Kim, S. H ., Fukushima, K ., Sly, J ., Miller, R. D ., Waymouth, R. M ., Yang, Y. Y . & Hedrick, J. L . A simple approach to stabilized micelles employing mikto-arm terpolymers and stereocomplexes with application in paclitaxel delivery. Biomacromolecules 10, 1460–1468 (2009).
Geihe, E. I ., Cooley, C. B ., Simon, J. R ., Kiesewetter, M. K ., Edward, J. A ., Hickerson, R. P ., Kaspar, R. L ., Hedrick, J. L ., Waymouth, R. M . & Wender, P. A . Designed guanidinium-rich amphipathic oligocarbonate molecular transporters complex, deliver and release siRNA in cells. Proc. Natl Acad. Sci. USA 109, 13171–13176 (2012).
Engler, A. C ., Wiradharma, N ., Ong, Z. Y ., Coady, D. J ., Hedrick, J. L . & Yang, Y. Y . Emerging trends in macromolecular antimicrobials to fight multi-drug-resistant infections. Nano Today 7, 201—222 (2012).
Fukushima, K ., Liu, S ., Wu, H ., Engler, A. C ., Coady, D. J ., Maune, H ., Pitera, J ., Nelson, A ., Wiradharma, N ., Venkataraman, S ., Huang, Y ., Fan, W ., Ying, J. Y ., Yang, Y. Y . & Hedrick, J. L . Supramolecular high-aspect ratio assemblies with strong antifungal activity. Nat. Commun. 4, 2861 (2013).
Silva, A. D ., Sundberg, L. K ., Ito, H ., Sooriyakumaran, R ., Allen, R. D . & Ober, C. K . A fundamental study on dissolution behavior of high-resolution molecular glass photoresists. Chem. Mater. 20, 7292–7300 (2008).
Tsuruta, T . Contemporary topics in polymeric materials for biomedical applications. Adv. Polym. Sci. 126, 1–51 (1996).
Newcomb, C. J ., Sur, S ., Ortony, J. H ., Lee, O.-S ., Matson, J. B ., Boekhoven, J ., Yu, J.-M ., Schatz, G. C . & Stupp, S. I . Cell death versus cell survival instructed by supramolecular cohesion of nanostructures. Nat. Commun. 5, 3321 (2014).
Fukushima, K . & Tanaka, M . Biocompatible and biodegradable polymers. Patent application pending JP2014 097237.
Hatakeyma, T ., Kasuga, H ., Tanaka, M . & Hatakeyama, H . Cold crystallization of poly(ethylene glcyol)-water sytems. Thermochim. Acta 465, 59–66 (2007).
Hatakeyma, T ., Tanaka, M . & Hatakeyama, H . Thermal properties of freezing bound water restrained by polysaccharides. J. Biomat. Sci. Polym. Ed. 21, 1865–1880 (2010).
Hatakeyma, T ., Tanaka, M . & Hatakeyama, H . Studies on bound water restrained by poly(2-methacryloyloxyethyl phosphorylcholine) (PMPC): comparison of the polysaccharides-water systems. Acta Biomater. 6, 2077–2082 (2010).
Hatakeyma, T ., Kishi, A . & Tanaka, M . Comparison of measurement techniques for the identification of bound water restrained by polymers. Thermochim. Acta 532, 159–163 (2012).
Hayashi, T ., Tanaka, Y ., Koide, Y ., Tanaka, M . & Hara, M . Mechanism underlying bioinertness of self-assembled monolayers of oligo(ethyleneglycol)-terminated alkanethiols on gold: protein adsorption, platelet adhesion, and surface forces. Phys. Chem. Chem. Phys. 14, 10194–10206 (2012).
Hirata, T ., Matsuno, H ., Tanaka, M . & Tanaka, K . Surface segregation of poly(2-methoxyethyl acrylate) in a mixture with poly(methyl methacrylate). Phys. Chem. Chem. Phys. 13, 4928–4934 (2011).
Morita, S ., Tanaka, M ., Kitagawa, K . & Ozaki, Y . Hydration structure of poly(2-methoxyethyl acrylate): comparison with a model monomer. J. Biomat. Sci. Polym. Ed. 21, 1925–1935 (2010).
Mochizuki, A ., Kishi, A . & Tanaka, M . Comparative study on water structures in polyHEMA and polyMEA by XRD-DSC simultaneous measurement. J. Appl. Poly. Sci. 111, 476–481 (2009).
Tanabe, A ., Morita, S ., Tanaka, M ., Noda, I . & Ozak, Y . Multivariate curve resolution analysis on multi-component water sorption process into a poly(2-methoxyethyl acrylate) film. Appl. Spec. 62, 46–50 (2008).
Kitano, H ., Nagaoka, K ., Tada, S ., Gemmei-Ide, M . & Tanaka, M . Structure of water incorporated in amphoteric polymer thin films as revealed by FT-IR spectroscopy. Macromol. Biosci. 8, 77–85 (2008).
Morita, S ., Tanaka, M ., Noda, I . & Ozaki, Y . Phase angle description of perturbation correlation analysis and its application to time-resolved IR spectra. Appl. Spec. 61, 867–872 (2007).
Kitano, H ., Ichikawa, K ., Fukuda, M ., Mochizuki, A . & Tanaka, M . The structure of water sorbed to poly(methyl methacrylate) film as examined by FT-IR spectroscopy. J. Colloid Surface Sci. 242, 133–140 (2001).
Ichikawa, K ., Mori, T ., Kitano, H ., Fukuda, M ., Mochizuki, A . & Tanaka, M . A FT-IR study on the sorption of water to various kinds of polymer thin films. J. Polym. Sci. B Polym. Phys. 39, 2175–2182 (2001).
Tanaka, M . Design of novel 2D and 3D bio-interfaces using self-organization to control cell behavior. Biochim. Biophys. Acta 1810, 251–258 (2011).
Oda, Y ., Horinouchi, A ., Kawaguchi, D ., Matsuno, H ., Kanaoka, S ., Aoshima, S . & Tanaka., K . Effect of side-chain carbonyl groups on the interface of vinyl polymers with water. Langmuir 30, 1215–1219 (2014).
Morita, S . & Tanaka, M . Effect of sodium chloride on hydration structures of PMEA and p(MPC-r-BMA). Langmuir 30, 10698–10703 (2014).
Acknowledgements
This work is supported by Grants-in-Aid and Special Coordination Funds for Promoting Science and Technology of Ministry of Education, Culture, Sports, Science and Technology, Japan (KAKENHI). We greatly acknowledge the financial support from Funding Program for Next Generation World-Leading Researchers (NEXT Program, Japan).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tanaka, M., Sato, K., Kitakami, E. et al. Design of biocompatible and biodegradable polymers based on intermediate water concept. Polym J 47, 114–121 (2015). https://doi.org/10.1038/pj.2014.129
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pj.2014.129
This article is cited by
-
Enhancing the functionality of biodegradable Mg–Zn–Mn alloys using poly(lactic) acid (PLA) coating for temporary implants
Journal of Coatings Technology and Research (2024)
-
Advances in Wireless, Batteryless, Implantable Electronics for Real-Time, Continuous Physiological Monitoring
Nano-Micro Letters (2024)
-
Synthesis of antibacterial poly((2-methylsulfinyl)ethyl acrylate-co-acrylic acid)-based electrospun nanofibers
Polymer Bulletin (2023)
-
Water-soluble polymer micelles formed from amphiphilic diblock copolymers bearing pendant phosphorylcholine and methoxyethyl groups
Polymer Journal (2021)
-
Design and fabrication of flexible DNA polymer cocoons to encapsulate live cells
Nature Communications (2019)