Abstract
The effects of cyanobiphenyl (CB) homologue molecules on the electro-optical properties of low-molecular-weight liquid crystal blue phase (BP) and polymer-stabilized BPs are investigated using chiral nematic liquid crystal mixtures that include CB molecules. The driving voltage and switching hysteresis of BPs were found to increase by polymer stabilization of the BP. It was also confirmed that the electro-optical rising time and Kerr coefficient of BPs vary with the molecular parity, as determined by the alkyl chain length of the n-CB homologue molecule dissolved in a chiral nematic liquid crystal mixture.
Similar content being viewed by others
Introduction
Blue phases (BPs) are liquid crystal (LC) phases that appear in the temperature range between the chiral nematic and isotropic liquid phases for chiral nematic LCs under high chirality conditions. BPs have a three-dimensional cubic structure with lattice periods of several hundred nanometres in length1, 2, 3 and therefore exhibit selective Bragg reflections in the visible and ultraviolet (UV) wavelength ranges. BPs are optically isotropic due to the cubic symmetry of their molecular arrangement in the absence of an electric field. However, BPs become optically anisotropic due to the unidirectional anisotropy of molecular alignment in an applied electric field, with a response time of several hundred microseconds due to the Kerr effect.4 For practical applications, although BPs hold potential as fast light modulators,5, 6, 7, 8, 9 their narrow temperature range of less than a few degrees Kelvin has always been a problem.
Recently, the polymer-stabilized (PS) BP, with a wide temperature range of more than 60 K that includes room temperature, was reported by Kikuchi et al.10 as a high-performance optical switch with a fast electro-optical response time of the order of 10−4 s. However, the polymer stabilization of BP resulted in high driving voltage and large switching hysteresis compared with low-molecular-weight (LM) BP without polymer stabilization. To achieve low driving voltages in PS-BP, device structures and BP materials have been studied extensively to improve their performance. In terms of device structure, an implementing protrusion electrode11, 12 and vertical field switching13 as well as BP materials with a large Kerr coefficient14, 15 and materials exhibiting amorphous BP III16, 17 have been reported.
To date, there are few reports about the relationship between the electro-optical properties and chemical structures of LM-BP and PS-BP for rod-like chiral nematic LCs.18 In this study, the driving voltage and switching hysteresis of LM-BP and PS-BP were investigated using four types of n-CB homologue chiral nematic LC mixtures. Furthermore, the electro-optical response time and Kerr coefficient of LM-BP and PS-BP were evaluated for the same four types of n-CB homologue chiral nematic LC mixtures.
Experimental procedure
Sample preparation
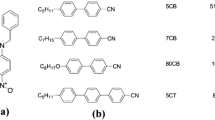
4-cyano-4′-n-alkyl biphenyl (n-CB) homologues (Aldrich, St Louis, MO, USA) and JC1041XX (JNC Co. Ltd., Tokyo, Japan) were used as host nematic LC materials, and ISO-(6OBA)2 was used as a chiral dopant, as shown in Figure 1. Four types of n-CB homologue LCs served as n-CB compounds with n=5, 6, 7 and 8. Here, JC1041XX is a mixture of fluorinated liquid crystalline molecules and was used to obtain stable BPs and PS-BPs. For polymer stabilization of BP, RM257 (Merck, Whitehouse Station, NJ, USA) and dodecyl acrylate (Aldrich) were used as reactive monomers, and 2, 2-dimethoxy-2-phenyl-acetophenone (DMPAP, Aldrich) was used as a photoinitiator, as shown in Figure 1.
In the case of LM-BP without polymer stabilization, four types of chiral nematic LC mixtures were prepared in ratios of n-CB:JC1041XX:ISO-(6OBA)2=46.25:46.25:7.5 by weight percentage. In Addition, in the case of PS-BP, four types of chiral nematic LC mixtures were prepared in ratios of n-CB:JC1041XX:ISO-(6OBA)2:RM257:dodecyl acrylate:DMPAP=42.6:42.6:7.5:3.5:3.5:0.3 by weight percentage. Each n-CB chiral nematic LC mixture was placed in the vacant space of a 10 μm gap sandwich cell without surface treatment. In particular, for fabrication of the PS-BP cell, four types of LC mixtures were irradiated with UV light with maximum wavelength of 365 nm when the four types of LC mixtures were kept at a BPI. UV light irradiation was maintained for 15 min, with light intensity of 600 mW cm−2.
Evaluation of BP temperature range for each n-CB homologue chiral nematic LC mixture
Each temperature range for the BPs was characterized by polarizing optical microscope (POM, Nikon, Tokyo, Japan) observations of texture. The optical textures of the four types of LM-BP and PS-BP were observed by a POM equipped with a hot stage calibrated to an accuracy of ±0.1 K (Linkam LK-600PM, Guildford, UK) under crossed Nicols. The cooling and heating ratios of the cell were each set to 1 K min−1, and the lower observable limit temperature of POM was 253 K on cooling.
Measurement of elastic constants for each host nematic LC mixture and chiral pitch for each chiral nematic LC mixture
As the measurement method for elastic constants (K11, K22 and K33) of chiral nematic LC is not yet established, they were evaluated using nematic LC mixtures without a chiral dopant. The four types of host nematic LC mixtures were prepared as (5CB/JC1041XX), (6CB/JC1041XX), (7CB/JC1041XX) and (8CB/JC1041XX) in 50:50 weight percentages. The splay (K11) and bend elastic constants (K33) were evaluated based on a relationship between the electric capacitance and applied voltage for the four types of (n-CB/JC1041XX) nematic LC mixtures without chiral dopants in a 10-μm-thick twisted nematic sandwich cell using EC-1 (Toyo Technica Co. Ltd., Tokyo, Japan). During the measurement of K11 and K33, dielectric constants (Δɛ) of each host nematic LC were evaluated simultaneously. The measuring temperature was Tc –10 K for the four types of n-CB nematic LC mixtures. Furthermore, K22 was calculated from the measured K11 and K33.19 The average elastic constant was estimated by the arithmetic average of K11, K22 and K33. The chiral pitch for the four types of n-CB chiral LC mixtures was evaluated by measurement of the width of the disclination line on POM observation using the wedge cell with tan θ=0.0083. The chiral dopant concentration of each chiral nematic LC mixture was 0.4 wt% (n-CB:JC1041XX:ISO-(6OBA)2=49.8:49.8:0.4). The measurement temperature was Tc−10 K for each chiral nematic LC mixture, where Tc was the phase transition temperature from the chiral nematic to isotropic phase for each chiral nematic LC mixture.
Measurement of electro-optical properties of LM-BP and PS-BP for four types of n-CB homologue chiral nematic LC mixtures
A sandwich-type in-plane switching electrode cell was prepared to measure the electro-optical properties of BP-LC mixtures. The top bare glass was not surface treated, and the bottom Cr-coated glass was patterned with a distance of 10 μm in plane. The two glasses were attached by a UV light-curing adhesive and kept in a 10 μm gap with a PET film as a spacer. The chiral nematic LC mixtures were injected into vacant spaces of the sandwich-type electrode cell at the isotropic phase to prevent flow alignment. BP polymer stabilization was accomplished by UV irradiation of the n-CB homologue chiral nematic LC mixtures, while the LC maintained a BPI. A He–Ne laser (λ=633 nm) was positioned perpendicular to the sandwich cell. The transmitted light through the cell was detected by a photodiode (New Focus 1621M, Santa Clara, CA, USA) and recorded with a digital storage oscilloscope (LeCroy WP950, Chestnut Ridge, NY, USA). The driving voltage of each LM-BP and PS-BP was defined as the voltage at the maximum transmittance during voltage increase. The switching hysteresis of each LM-BP and PS-BP was defined as the voltage difference between voltage increase and decrease at 50% transmittance.
The Kerr coefficient of each LM-BP and PS-BP was evaluated by the extended Kerr model20, 21 through measurement of the relationship between the transmittance and square of applied voltage. The measurements of driving voltage, switching hysteresis, response time and Kerr coefficient were performed at Tc−0.5 K for LM-BP and Tc−10 K for PS-BP.
The electro-optical response time was evaluated by measurement of the time during which the transmittance changed from 10 to 90% with voltage on (rising time) or from 90 to 10% with voltage off (decay time) in the cell. When the electro-optical response time was measured, a squared AC electric voltage with frequency of 1.0 kHz was applied for 1 s over 30 times to the cell.
Results and discussion
BP temperature ranges of LM-BP and PS-BP for four types of n-CB homologue chiral nematic LC mixtures
Table 1 shows the phase transition temperature of (a) LM-BP and (b) PS-BP for the four n-CB chiral nematic LC mixtures on heating. The ΔTBPs for four types of LM-BP were small, ranging from 1 to 1.6 K, whereas ΔTBPs for four types of PS-BP were over a wide range of more than 60 K. For the four types of PS-BP, in particular, the phase transition between the BP and chiral nematic phases could not be observed, even though the temperature was lowered by 253 K. Here, 253 K was the lower observable temperature limitation of our POM equipped with a hot stage but was not the phase transition temperature between the BP and chiral nematic phases. However, we observed wide BP temperature ranges of more than 60 K through the polymer stabilization of four types of n-CB homologue chiral nematic LC mixtures.
Electro-optical properties of LM-BP and PS-BP for four types of n-CB homologue chiral LC mixtures
Figure 2 shows the experimental results of light transmittance against applied voltage of LM-BP and PS-BP for four types of n-CB homologue chiral nematic LC mixtures. Table 2 shows the driving voltage, switching hysteresis and Kerr coefficient estimated from Figure 2.
The driving voltage of the four types of LM-BP had lower voltages (83.5 V for 5CB, 97.5 V for 6CB, 80.8 V for 7CB and 95.5 V for 8CB) than the four types of PS-BP (92.5 V for 5CB, 105.2 V for 6CB, 90.5 V for 7CB and 107.2 V for 8CB). From Table 2 and Figure 2, we found that the driving voltages of LM-BPs and PS-BPs varied with the molecular parity of n-CB, and PS-BP had a higher driving voltage than LM-BP. The increase in driving voltage of PS-BP can be understood by comparing the series-connected dielectric model of the composite of LC and polymer on application of an AC electric field reported by Miyamoto et al.,22 as shown in Figure 3. Using this model, the applied electric field E could be approximately written by Equation (1), where ϕ is the volume ratio of LC in the composite of polymer and LC.

ELC and EP are electric fields distributed to the LC and polymer regions, respectively. In Addition, the ratio of the electric fields applied to the LC and polymer could be represented as the ratio of the moduli of impedance of the LC and polymer, ZLC and ZP, as shown in Equation (2).

Therefore, for the PS-BP-containing polymer, the applied electric field is divided into the LC and polymer, whereas for LM-BP, the applied electric field is imposed to only LC on the basis of Equation (1) and Equation (2). As a result, the driving voltage for maximum transmittance of PS-BP is higher than that of LM-BP because |ZP| is larger than |ZLC|. Furthermore, anchoring of LC molecules at the LC/polymer interface is a possible cause of the higher driving voltage of PS-BP.
In the case of switching hysteresis, those of PS-BPs ranged from 3.8 to 5.1 V, whereas LM-BPs showed smaller hysteresis from 0.2 to 0.4 V. This result implies that LM-BPs without polymer show nearly elastic behaviour of only LC in an electric field, whereas PS-BPs with polymer show a combination of the viscoelastic behaviour of polymers and elastic behaviour of LC in an electric field. As a result, the switching hysteresis of PS-BPs is larger than those of LM-BPs. In addition, we should consider that the switching hysteresis of PS-BPs is affected by the interfacial interaction of the LC and polymer. However, we could not find this relationship among the species of n-CB homologue LC mixtures, perhaps because the switching hysteresis of PS-BP is governed by several factors, including an anchoring force between polymer and LC and polymer network structure as well as the chemical structure of host nematic LCs.
Interestingly, the Kerr coefficients of both LM-BPs and PS-BPs prepared with an odd alkyl-numbered n-CB, 5CB and 7CB, were larger than those with an even alkyl-numbered n-CB, 6CB and 8CB, as shown in Table 2. Namely, the Kerr coefficients of both LM-BPs and PS-BPs were found to vary with the molecular parity of the n-CB homologue LC. Furthermore, the Kerr coefficients were inversely proportional to the square of the driving voltages of the LM-BPs and PS-BPs of n-CB homologue chiral nematic LCs. These results quantitatively showed similar tendencies to previous research results14 that the driving voltage is inversely proportional to the square root of the Kerr coefficient.
Table 3 shows birefringence (Δn), dielectric anisotropy (Δɛ) and average elastic constants (k) of host nematic LC mixtures and chiral pitch (p) of n-CB homologue chiral LC mixtures at Tc−10 K. In the table, Δɛ, p and k were the measured values at Tc−10 K, and Δn was the reference value from the LC database (Version 4.7; Volkmar Vill). From the Gerber model,6 the Kerr coefficient is approximately proportional to Δn·Δɛ·p2/k. However, our measured Kerr coefficients did not follow this relationship from the Gerber model but were only dependent on the Δn·Δɛ term in n-CB homologue LM-BPs and PS-BPs.
Figure 4 shows the experimental results of rising and decay times of (a) LM-BP and (b) PS-BP for four types of n-CB homologues. Although the rising time decreased when ‘n’ was odd and increased when ‘n’ was even, the decay time was rarely dependent on the number of alkyl chains for the four types of (a) LM-BP and (b) PS-BP LC, as shown in Figure 4. Namely, the rising times of LM-BP and PS-BP change with molecular parity, whereas the decay times rarely change with molecular parity for both LM-BP and PS-BP.
Figure 5 shows the relationship between the response time and Kerr coefficient of (a) LM-BP and (b) PS-BP for the four types of n-CB homologue LC mixtures. Interestingly, the rising time was more strongly dependent on the Kerr coefficient than was the decay time of both LM-BPs and PS-BPs, based on the results in Figures 5a and b.
Conclusions
The driving voltage and switching hysteresis of BPs were increased by polymer stabilization for n-CB homologue chiral nematic LCs.
The electro-optical rising time and Kerr coefficient of BPs were found to change with molecular parity, as determined by the alkyl chain length of n-CB homologue chiral nematic LC mixtures.
References
De Gennes, P. G. & Prost, J. The Physics of Liquid Crystals, (Clarendon Press, Oxford, 1995).
Crooker, P. P. Chirality in Liquid Crystals, (Springer, New York, 2001).
Higashiguchi, K., Yasui, K. & Kikuchi, H. Direct Observation of polymer-stabilized blue phase I structure with confocal laser scanning microscope. J. Am. Chem. Soc. 130, 6326–6327 (2008).
Kerr, J. A new relation between electricity and light: dielectrified media birefringent. Philos. Mag. 50, 337–348 (1875).
Dmitrienko, V. E. Electro-optic effects in blue phases. Liq. Cryst. 5, 847–851 (1989).
Gerber, P. R. Electro-optical effects of a small-pitch blue-phase system. Mol. Cryst. Liq. Cryst. 116, 197–206 (1985).
Coles, H. G. & Gleeson, H. F. Electric-field induced phase-transitions and color switching in the blue phases of chiral nematic liquid-crystals. Mol. Cryst. Liq. Cryst. 167, 213–225 (1989).
Heppke, G., Jerome, B., Kitzerow, H. S. & Pieranski, P. Electrostriction of the cholesteric blue phases BPI and BPII in mixtures with positive dielectric anisotropy. J. Phys. 50, 2991–2998 (1989).
Wenyi, C., Antonio, M., Peter, P. M. & Bahman, T. Lasing in a three-dimensional photonic crystal of the liquid crystal blue phase II. Nat. Mater. 1, 111–113 (2002).
Kikuchi, H., Yokota, M., Hisakado, Y., Yang, H. & Kajiyama, T. Polymer-stabilized liquid crystal blue phases. Nat. Mater. 1, 64–68 (2002).
Rao, L., Ge, Z., Wu, S. T. & Lee, S. H. Low voltage blue-phase liquid crystal displays. Appl. Phys. Lett. 95, 231101 (2009).
Kim, M., Kim, M. S., Kang, B. G., Kim, M. K., Yoon, S., Lee, S. H., Ge, Z., Rao, L., Gauza, S. & Wu, S. T. Wall-shaped electrodes for reducing the operation voltage of polymer-stabilized blue phase liquid crystal displays. J. Phys. D: Appl. Phys. 42, 235502 (2009).
Cheng, H. C., Yan, J., Ishinabe, T. & Wu, S. T. Vertical field switching for blue-phase liquid crystal device. Appl. Phys. Lett. 98, 261102 (2011).
Rao, L., Yan, J., Wu, S. T., Yamamoto, S. & Haseba, Y. Large Kerr constant polymer-stabilized blue phase liquid crystal. Appl. Phys. Lett. 98, 081109 (2011).
Chen, Y., Xu, D., Wu, S. T., Yamamoto, S. & Haseba, Y. A low voltage and submillisecond-response polymer-stabilized blue phase liquid crystal. Appl. Phys. Lett. 102, 141116 (2013).
Yoshizawa, A., Kamiyama, M. & Hirose, T. Amorphous blue phase III exhibiting submillisecond response switching at room temperature. Appl. Phys. Express. 4, 101701 (2011).
Kogawa, Y., Hirose, T. & Yoshizawa, A. Biphenyl derivative stabilizing blue phases. J. Mater. Chem. 21, 19132–19137 (2011).
Mizunuma, T., Oo, T. N., Nagano, Y., Ma, H., Haseba, Y., Higuchi, H., Okumura, Y. & Kikuchi, H. Electro-optical properties of polymer-stabilized blue phase with different monomer combination and concentration. Opt. Mater. Express. 1, 1561–1568 (2011).
Schadt, M. & Helfrich, W. Voltage dependent optical activity of a twisted nematic liquid crystal. Appl. Phys. Lett. 18, 127–128 (1971).
Yan, J., Cheng, H. C., Gauza, S., Li, Y., Jiao, M., Rao, L. & Wu, S. T. Extended Kerr effect of polymer-stabilized blue-phase liquid crystals. Appl. Phys. Lett. 96, 071105 (2010).
Yan, J., Jiao, M., Rao, L. & Wu, S. T. Direct measurement of electric-field-induced birefringence in a polymer-stabilized blue-phase liquid crystal composite. Opt. Express 18, 11450–11455 (2010).
Miyamoto, A., Kikuchi, H., Kobayashi, S., Morimura, Y. & Kajiyama, T. Dielectric property-electrooptical effect relationships of polymer/liquid-crystal composite films. Macromolecules 24, 3915–3920 (1991).
Acknowledgements
We thank JNC Co. LTD for providing the nematic mixture JC1041XX. This work was supported by the IT R&D programme of MKE/KEIT (10041596, Development of Core Technology for TFT Free Active Matrix Addressing Colour Electronic Paper with Day and Night Usage). This work was also supported partially by a Grant-in-Aid for Scientific Research (A) (No. 25248021) from the Ministry of Education, Culture, Sports, Science and Technology, Japan (MEXT).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lim, G., Kikuchi, H. & Hong, SK. Effect of cyanobiphenyl homologue molecules on electro-optical properties in liquid crystal blue phases and polymer-stabilized blue phases. Polym J 46, 337–341 (2014). https://doi.org/10.1038/pj.2013.98
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pj.2013.98