Abstract
A novel amphiphilic graft copolymer composed of poly(γ-glutamic acid) (γ-PGA) as a hydrophilic backbone and dodecylamine (DOA) as a hydrophobic side chain (γ-PGA-DOA) was successfully synthesized by employing 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC) as the coupling reagent. The grafting degree was stoichiometrically controlled by adjusting the feeding amounts of DOA and EDC in the reaction system. γ-PGA-DOA, with a grafting degree of 55%, self-assembled via hydrophobic interactions and formed microparticles (MPs; diameter of 13±3 μm) in an aqueous solution. These MPs possessed functional carboxylic groups and could be further modified with compounds such as drugs, proteins and peptides, and thus have great potential as vaccine carriers.
Similar content being viewed by others
Introduction
Nanotechnology has been extensively exploited in the biological and medical fields to improve pharmacokinetic and therapeutic properties. To achieve the efficient delivery of compounds such as drugs, proteins and nucleotides to target organs, cells and the long blood circulation, nano- or micro-carriers have been employed.1, 2 In particular, polymeric carriers composed of amphiphilic block or graft copolymers have received considerable interest because of their attractive properties. These copolymers can form self-assembled structures, such as micelles, vesicles and spheres.3, 4, 5 In general, these copolymers possess hydrophobic moieties that encapsulate a hydrophobic drug as well as hydrophilic moieties that are responsible for the stabilization of the structure and biocompatibility. It is also promising that the physical properties of the carriers, such as their size, surface charge and hydrophobicity, can be precisely controlled by designing the copolymers.6, 7
Because of these properties, polymeric carriers have been eagerly investigated; however, the internal in vivo stability has remained a challenge for these delivery systems. To overcome this problem, chemical crosslinking is normally used to improve stability; however, the use of crosslinkers may cause undesirable interactions for the encapsulated cargo and may affect the biodegradability or biocompatibility of the delivery system.8 Therefore, the stabilization of polymeric carriers through hydrophobic interactions and hydrogen bonding is an alternative and attractive strategy.
Vesicles, liposomes, polymeric nanoparticles (NPs) and microparticles (MPs) are typical examples of carriers prepared via hydrophobic interactions and/or hydrogen bonds.9, 10, 11 These carriers have attracted significant attention by offering advantages such as the efficient delivery of compounds to target organs and reduced toxicity. Liposomes have received a considerable amount of interest over the past decades as pharmaceutical carriers.12 Liposomes are composed of biocompatible phospholipids and can encapsulate various compounds in their internal water compartment and membranes. Furthermore, the size, charge and composition of liposomes are easily controlled by changing the lipid mixture before liposome preparation.
Although liposomes are promising candidates for pharmaceutical carriers, they possess some drawbacks. The stability of liposomes is not high, and they are easily fused together.13 Liposomes are also easily eliminated from the blood and captured by the cells. Moreover, the preparation of liposomes requires additional energy, such as ultrasonication.14 In addition, self-assembly of thermodynamically stable, long-lived vesicles, NPs and MPs has been realized by employing amphiphilic copolymers.15 These carriers are biocompatible, biodegradable and easily modified in the preparation step. These carriers can co-encapsulate hydrophobic molecules within the hydrophobic moieties. Because of these properties, researchers have developed various types of carriers.15 However, these carriers are based on polymers without reactive functional groups; therefore, it is difficult to prepare functional carriers.
In a previous study, we prepared biodegradable NPs composed of poly(γ-glutamic acid) (γ-PGA) conjugated with L-phenylalanine ethyl ester (Phe) as the hydrophobic segment for the development of safe and effective NP-based vaccines.16 γ-PGA-graft-Phe (γ-PGA-Phe) with a grafting degree of greater than 50% formed monodispersed NPs in water because of their hydrophobic interactions.17 We demonstrated that the size of γ-PGA-Phe NPs was precisely controlled by adjusting the preparation conditions.18, 19 These NPs could efficiently and stably encapsulate various antigens and could deliver them to target organs and cells, resulting in the potent induction of antigen-specific immune responses.20, 21 Moreover, we reported that the size of γ-PGA-Phe NPs affected the interactions (uptake, degradation of antigens and intracellular localization) between cells and the antigen-encapsulated γ-PGA-Phe NPs.21 Furthermore, γ-PGA-Phe NPs possess functional carboxylic groups in their backbone, and various compounds were covalently immobilized onto the surfaces of the NPs.22 In addition, we demonstrated that when long alkyl chains were grafted onto the hydrophilic polyglycidol, the long alkyl side chains were hierarchically ordered by hydrophobic interactions and hydrogen bonding.23 Taken together, we hypothesized that the combination of these characteristics should lead to a novel carrier with functional and unique properties that conventional vaccine carriers do not possess and that this carrier should become a promising candidate for novel vaccine carriers.
In this study, we present the preparation of novel functional MPs via self-assembly of an amphiphilic copolymer composed of γ-PGA as the hydrophilic backbone and dodecylamine (DOA) as the hydrophobic side chain (γ-PGA-DOA). In addition, characterization of the amphiphilic γ-PGA-DOA was performed.
Experimental Procedure
Materials
Poly(γ-glutamic acid) (γ-PGA, Mw=480 000) was purchased from Wako Pure Chemical Industries (Osaka, Japan). Dehydrated dimethyl sulfoxide (DMSO) and DOA were purchased from Merck KGaA (Darmstadt, Germany). 4-(N,N-dimethylamino)pyridine, methanol and chloroform (CHCl3) were purchased from Sigma (St Louis, MO, USA). 1-Ethyl-3-(3-dimethylaminoproyl)-carbodiimide (EDC) was purchased from Tokyo Chemical Industry Co., Ltd (Tokyo, Japan).
Characterization methods
Proton nuclear magnetic resonance (1H NMR) spectra were recorded on a Bruker DPX-400 FT-NMR spectrometer at 400 MHz (Bruker, Rheinstetten, Germany). DMSO-d6 was used as the solvent. Size exclusion chromatography analysis was performed at 30 °C using an isocratic pump (Agilent 1100 series, Agilent, Santa Clara, CA, USA) and a refractive index detector (Wyatt Optilab DSP, Wyatt Technology, Dernbach, Germany). The eluting solvent was water (HPLC gradient grade, Roth) with 50 mM sodium hydrogen carbonate (Carl Roth, Karlsruhe, Germany). Methanol (p.a., Th. Geyer) was used as the internal standard at a flow rate of 1 ml min−1. Four PSS suprema columns filled with modified acrylate-copolymer-network particles were used. The length of the precolumn was 50 mm, and the other three separation columns had lengths of 300 mm. The diameter of each column was 8 mm; the diameter of the copolymer particles was 10 μm; and the nominal pore widths were 30, 1000 and 3000 Å, respectively. Calibration was achieved using narrowly distributed poly(ethylene glycol)/poly(ethylene oxide) standards. Fourier transform infrared (FT-IR) spectra were recorded using a Nicolet NEXUS 670 Fourier Transform IR spectrometer (Thermo Fisher Scientific, Waltham, MA, USA).
Partial hydrolysis of γ-PGA
To obtain γ-PGA with a lower molecular weight, the γ-PGA was hydrolyzed under alkaline condition. The starting material γ-PGA (Mw=480 000) was dissolved in sodium hydroxide (2 M), and the mixture was then stirred at 80 °C for 8 h. After cooling to room temperature, the pH of the solution was adjusted to 1.0 with hydrochloric acid. The mixture was maintained at 4 °C for 3 days, and the resulting precipitate was collected by centrifugation. The obtained white solid was lyophilized for 2 days, and the molecular weight was measured by size exclusion chromatography. The eluting solvent was water with 50 mM sodium hydrogen carbonate, and methanol was used as the internal standard at a flow rate of 1 ml min−1.
Synthesis of γ-PGA-DOA copolymer
The γ-PGA-DOA was synthesized using a coupling reaction between γ-PGA and DOA under a nitrogen atmosphere. γ-PGA (500 mg, 3.88 unit mmol) was dissolved in 20 ml of dehydrated DMSO at 40 °C. After the γ-PGA was dissolved, 20 ml of DMSO containing EDC (Code 1: 744 mg, 3.88 mmol; Code 2: 372 mg, 1.94 mmol) and 4-(N,N-dimethylamino)pyridine (DMAP) (Code 1: 238 mg, 1.94 mmol; Code 2: 119 mg, 0.97 mmol) was added to the solution, and then 20 ml of DMSO containing DOA (Code 1: 718 mg, 3.88 mmol; Code 2: 359 mg, 1.94 mmol) was added; the reaction was allowed to continue for 24 h at 40 °C. The product was then dialyzed against methanol for 2 days, collected by evaporating the methanol and analyzed by FT-IR and 1H NMR. To evaluate the self-assembly behavior of the γ-PGA-DOA, the γ-PGA-DOA was labeled with fluorescein isothiocyanate (FITC). The γ-PGA-DOA (7.6 μmol) was dissolved in DMSO, followed by the addition of EDC (1.5 μmol), DMAP (750 nmol) and FITC (1.5 μmol). The reaction was conducted at room temperature for 24 h, then dialyzed against water for 3 days and freeze-dried for 2 days. The FITC-labeled γ-PGA-DOA (FITC-γ-PGA-DOA) was used for the following experiment.
Preparation of MPs
γ-PGA-DOA or FITC-γ-PGA-DOA was dissolved in CHCl3 at a concentration of 10 mg ml−1 followed by the addition of water. After the CHCl3 was removed, the sample was observed using differential interference contrast microscopy, confocal fluorescence microscopy (DSU-IX81-SET, Olympus, Tokyo, Japan) and scanning electron microscopy (JSM-6701 F, JEOL, Tokyo, Japan).
Results and Discussion
The degradation of γ-PGA was controlled by the concentration of sodium hydroxide and the hydrolysis time. When 2 M sodium hydroxide was employed and the hydrolysis time was 8 h, the γ-PGA was degraded to a molecular weight of Mn=7600 g mol−1, as measured by size exclusion chromatography. In this study, γ-PGA with a molecular weight of Mn=7600 g mol−1 was employed as the starting material.
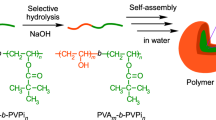
As illustrated in Scheme 1, the γ-PGA-DOA copolymer was synthesized via a coupling reaction between the terminal amine group of DOA and the carboxylic groups in γ-PGA using EDC at 40 °C for 24 h under a nitrogen atmosphere. The unconjugated DOA, unreacted EDC and by-products of the reaction were removed by dialysis against methanol. The synthesized γ-PGA-DOA was then characterized by its FT-IR spectra. γ-PGA has two characteristic peaks at 1640 and 1720 cm−1 because of carbonyl stretching vibrations, the first being due to the amide group and the other being due to the acid group (Figure 1a). After reacting with DOA, the intensity of the latter peak arising from the acid decreased, and the first peak from the amide group increased (Figure 1b).
This result supports that the γ-PGA-DOA copolymer was successfully synthesized in this reaction. However, quantitative analysis of the FT-IR spectra was difficult, because these two peaks partly overlapped, and it was difficult to separate them. Therefore, the grafting degree of DOA in γ-PGA-DOA was calculated from 1H NMR spectra (Figure 2c) using the integrals of the methine protons (4.10 p.p.m., c-CH) in the γ-PGA backbone (Figure 2b) and the ethyl peak (3.00 p.p.m., f-CH2) of the DOA (Figure 2a). The grafting degree of DOA to γ-PGA was 91% (Code 1) and 55% (Code 2) and was controlled stoichiometrically by adjusting the feeding ratio of DOA and EDC to γ-PGA (Table 1). We employed the γ-PGA-DOA with a 55% grafting degree for the following experiments.
The solubilities of the γ-PGA, DOA and γ-PGA-DOA copolymer in water (50 mM NaHCO3) and several common organic solvents were investigated. γ-PGA is known to be soluble in water and DMSO, whereas DOA can be dissolved in tetrahydrofuran, dichloromethane, CHCl3 and certain other organic solvents (Table 2). However, the solubility of the γ-PGA-DOA copolymer was different from that of γ-PGA or DOA; it dissolved in DMSO, dimethylformamide and 1,1,1,3,3,3-hexafluoro-2-propanol. γ-PGA-DOA dissolved in the 50 mM NaHCO3 solution because it represents an alkaline condition. However, γ-PGA-DOA did not dissolve in pure water. This difference was due to the hydrophilic/hydrophobic balance. Because γ-PGA-DOA is an amphiphilic polymer, it dissolved in solvents in which γ-PGA did not. These results confirm that the reaction did occur because the solubility of the copolymer changed.
For the preparation of MPs, various methods have been developed, such as solvent displacement and the emulsion method.15, 24 The amphiphilic copolymer is first dissolved in a common solvent, which is usually an organic solvent, followed by the addition of water. The hydrophobic groups in the copolymer tend to aggregate and form MPs when the organic solvent is removed. In this study, the MPs were prepared via the emulsion method employing CHCl3 as the organic solvent. γ-PGA-DOA was dissolved in CHCl3, and then the organic solvent was added into water. When the water content was low (the volume ratio of the polymer solution to water was 1:20), the γ-PGA-DOA copolymer self-assembled and formed MPs (Figure 3a). The internal structure of the MPs was evaluated by employing FITC-γ-PGA-DOA. The MPs composed of γ-PGA-DOA exhibited a packed structure, which suggests that the formation of MPs was coacervation (Figure 3b). The diameter of the MPs measured by differential interference contrast microscopy was 13±3 μm (n=50) and exhibited a narrow size distribution (Figure 3c). The MPs were further evaluated by scanning electron microscope observation (Figure 3d). The diameter was 6.1±1.6 μm (n=50), which was smaller than those in water. This finding suggests that water may have existed inside the MPs and that the MPs contracted when they were dried to remove water for scanning electron microscope observation.
Optical (a) and fluorescence (b) images of MPs composed of γ-PGA-DOA. The scale bar represents 20 μm. (c) The size distribution of microparticles (n=50). (d) Scanning electron microscope image of MPs. Scale bar represents 20 μm. The inset is a high-magnification image. Scale bar represents 5 μm. A full color version of this figure is available at Polymer Journal online.
We evaluated the stability of MPs in ultrapure water, which revealed that these MPs retained their structures for more than 1 week, suggesting that the MPs are stable and could be a great candidate for vaccine carriers. The formation of MPs was due to the hydrophilic/hydrophobic balance. Because the grafting degree of DOA was 55%, the γ-PGA-DOA copolymer possessed both hydrophilic carboxylic groups and hydrophobic dodecyl groups. Although the structure of the MPs is still not clear, we believe that the aliphatic alkyl chains formed hydrophobic domains inside the MPs and that the hydrophilic carboxylic groups in the γ-PGA backbone faced the water phase and thus stabilized the structure. The critical aggregation concentration of γ-PGA-DOA was 5 μg ml−1 when critical aggregation concentration was evaluated by employing a fluorescent dye (data not shown).
To use these MPs as vaccine carriers, they should be fabricated from biocompatible and biodegradable compounds. γ-PGA is edible, biodegradable and composed of naturally occurring D- and L-glutamic acids γ-linked together through amide bonds.25 DOA has also been reported to be biodegradable26 and has been studied as a skin permeation enhancer that can serve as a transdermal delivery system.27, 28 According to these reports, γ-PGA-DOA should be biocompatible, biodegradable and should possess great potential as a vaccine carrier. In addition, the size and morphology are important factors that control the behavior of the encapsulated antigen and carriers in our body and cells. In a previous study, we demonstrated that the size of γ-PGA-Phe NPs was precisely controlled by adjusting the salt concentration.18 We also discovered that γ-PGA-Phe NPs could form unimer NPs by adjusting the grafting degree of the hydrophobic segment.19
These findings suggest that the size of the MPs could be controlled using these techniques. A study on the biocompatibility, biodegradability and control over the size of the MPs is now in progress and should be reported elsewhere. Furthermore, allylamine was quantitatively grafted to γ-PGA (unpublished data), which suggests that these MPs may be able to be further functionalized with drugs, proteins and peptides by click reactions. Further investigation is necessary to determine the structure of the MPs, their stability in physiological conditions, the encapsulation efficiency of antigens, the functionalization and the biocompatibility of these novel carriers. These results nevertheless demonstrate that novel functional MPs were successfully prepared by hydrophobic interactions employing the γ-PGA-DOA copolymer.
Conclusions
In conclusion, the amphiphilic graft copolymer γ-PGA-DOA was successfully synthesized. The grafting degree of DOA was controlled stoichiometrically by adjusting the feeding amounts of DOA and EDC in the reaction system. This copolymer could form MPs due to the amphiphilic property of the γ-PGA-DOA copolymer. These MPs consist of a large number of carboxylic groups on the MPs. Therefore, these MPs should be potentially applicable for further functionalization, such as in the immobilization of bioactive compounds like proteins and peptides. Further research into the applications of MPs as vaccine carriers is now in progress.

Synthesis of the γ-PGA-DOA copolymer.
References
Cruz, J. L., Tacken, J. P., Fokkink, R., Joosten, B., Stuart, C. M., Albericio, F., Torensma, R. & Figdor, G. C. Targeted PLGA nano- but not microparticles specifically deliver antigen to human dendritic cells via DC-SIGN in vitro. J. Control Release 144, 118–126 (2010).
Kanchan, V. & Panda, K. A. Interactions of antigen-loaded polylactide particles with macrophages and their correlation with the immune response. Biomaterials 28, 5344–5357 (2007).
Ozawa, Y., Sawada, S., Morimoto, N. & Akiyoshi, K. Self-assembled nanogel of hydrophobized dendritic dextrin for protein delivery. Macromol. Biosci. 9, 694–701 (2009).
Yokoyama, M., Kwon, S. G., Okano, T., Sakurai, Y., Seto, T. & Kataoka, K. Preparation of micelle-forming polymer-drug conjugates. Bioconjugate Chem. 3, 295–301 (1992).
Brewer, M. J., Pollock, J. G. K., Tetley, L. & Russell, G. D. Vesicle size influences the trafficking, processing, and presentation of antigens in lipid vesicles. J. Immunol. 173, 6143–6150 (2004).
Dash, C. B., Réthoré, G., Monaghan, M., Fitzgerald, K., Gallagher, W. & Pandit, A. The influence of size and charge of chitosan/polyglutamic acid hollow spheres on cellular internalization, viability and blood compatibility. Biomaterials 31, 8188–8197 (2010).
Chiu, L. Y., Ho, C. Y., Chen, M. Y., Peng, F. S., Ke, J. C., Chen, J. K., Mi, L. F. & Sung, W. H. The characteristics, cellular uptake and intracellular trafficking of nanoparticles made of hydrophobically-modified chitosan. J. Control Release 146, 152–159 (2010).
Chen, S., Cheng, X. S. & Zhuo, X. R. Self-assembly strategy for the preparation of polymer-based nanoparticles for drug and gene delivery. Macromol. Biosci. 11, 576–589 (2011).
Mundargi, C. R., Babu, R. V., Rangaswamy, V., Patel, P. & Aminabhavi, M. T. Nano/micro technologies for delivering macromolecular therapeutics using poly(D,L-lactide-co-glycolide) and its derivatives. J. Control Release 125, 193–209 (2008).
Song, S., Zheng, Q., Song, A. & Hao, J. Self-assembled aggregates originated from the balance of hydrogen-bonding, electrostatic, and hydrophobic interactions. Langmuir 28, 219–226 (2012).
Molla, R. M. & Ghosh, S. Hydrogen-bonding-mediated vesicular assembly of functionalized naphthalene-diimide-based bolaamphiphilic and guest-induced gelation in water. Chem. Eur. J. 18, 9860–9869 (2012).
Torchilin, P. V. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 4, 145–160 (2005).
Saha, R., Verma, K. P., Mitra, K. R. & Pal, K. S. Structural and dynamical characterization of unilamellar AOT vesicles in aqueous solutions and their efficacy as potential drug delivery vehicle. Colloid. Surf. B 88, 324–353 (2011).
Maria, A., Ribeiro, C., Yoshida, S. L., Sesso, A. & Chaimovich, H. Permeabilities and stabilities of large dihexadecylphosphate and dioctadecyldimethylammonium chloride vesicles. J. Colloid Interface Sci. 100, 433–443 (1984).
Berkland, C., King, M., Cox, A., Kim, K. K. & Pack, W. D. Precise control of PLG microsphere size provides enhanced control of drug release rate. J. Control Release 82, 137–147 (2002).
Matsusaki, M., Hiwatari, K., Higashi, M., Kaneko, T. & Akashi, M. Stably-dispersed and surface-functional bionanoparticles prepared by self-assembling amphipathic polymers of hydrophilic poly(γ-glutamic acid) bearing hydrophobic amino acids. Chem. Lett. 33, 398–399 (2004).
Akagi, T., Baba, M. & Akashi, M. Preparation of nanoparticles by the self-organization of polymers consisting of hydrophobic and hydrophilic segments: potential applications. Polymer (Guildf). 48, 6729–6747 (2007).
Kim, H., Akagi, T. & Akashi, M. Preparation of size tunable amphiphilic poly(amino acid) nanoparticles. Macromol. Biosci. 9, 825–932 (2009).
Akagi, T., Piyapakorn, P. & Akashi, M. Formation of unimer nanoparticles by controlling the self-association of hydrophobically modified poly(amino acid)s. Langmuir 28, 5249–5256 (2012).
Akagi, T., Wang, X., Uto, T., Baba, M. & Akashi, M. Protein direct delivery to dendritic cells using nanoparticles based on amphiphilic poly(amino acid) derivatives. Biomaterials 28, 3427–3436 (2007).
Akagi, T., Shima, F. & Akashi, M. Intracellular degradation and distribution of protein-encapsulated amphiphilic poly(amino acid) nanoparticles. Biomaterials 32, 4959–4967 (2011).
Akagi, T., Kaneko, T., Kida, T. & Akashi, M. Multifunctional conjugation of proteins on/into bio-nanoparticles prepared by amphiphilic poly(γ-glutamic acid). J. Biomater. Sci. Polym. Ed. 17, 875–892 (2006).
Backes, M., Messager, L., Mourran, A., Keul, H. & Moeller, M. Synthesis and thermal properties of well-defined amphiphilic block copolymers based on polyglycidol. Macromolecules 43, 3238–3248 (2010).
Tian, B., Tao, X., Ren, T., Weng, Y., Lin, X., Zhang, Y. & Tang, X. Polypeptide-based vesicles: formation, properties and application for drug delivery. J. Mater. Chem. 22, 17404–17414 (2012).
Shih, L. I. & Van, T. Y. The production of poly-(γ-glutamic acid) from microorganisms and its various applications. Bioresour. Technol. 79, 207–225 (2001).
Ginkel, G. C., Pomper, A. M., Stroo, A. C. & Kroon, M. G. A. Biodegradation of fatty amides: utilization of the alkyl chain by isolated micro-organisms. Tenside Surf. Deterg 32, 355–359 (1995).
Kim, K. M., Zhao, H., Lee, H. C. & Kim, D. D. Formulation of a reservoir-type testosterone transdermal delivery system. Int. J. Pharm. 219, 51–59 (2001).
Bian, S., Doh, J. H., Zheng, J., Kim, S. J., Lee, H. C. & Kim, D. D. In vitro evaluation of patch formulations for topical delivery of gentisic acid in rats. Eur. J. Pharm. Sci. 18, 141–147 (2003).
Acknowledgements
This work was supported by the Japan Society for the Promotion of Science (JSPS), Japanese-German Graduate Externship, and Deutsche Forschungsmeinschaft within the project ‘Selectivity in Bio- and Chemoselectivity’. This work was partially supported by a Grant-in-Aid for Scientific Research (S) from the Ministry of Education, Culture, Sports, Science and Technology (23225004).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Shima, F., Schulte, B., Keul, H. et al. Preparation of microparticles composed of amphiphilic poly(γ-glutamic acid) through hydrophobic interactions. Polym J 46, 184–188 (2014). https://doi.org/10.1038/pj.2013.74
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pj.2013.74