Abstract
A pH-responsive amphiphilic poly(N-isopropylacrylamide) (PNIPAm) was synthesized by grafting on a peptide (Leu-Lys)8. The grafted content of the copolymer was 12 mol%. We investigated the pH- and thermo-induced conformational and morphological changes of the grafted copolymer and found small globular aggregates below and above the coil-to-globule transition temperature of the main chain under acidic conditions. However, under basic conditions, the peptide graft chains formed a stable β-sheet structure above the transition temperature. Under basic conditions and at high temperature, the main chains formed a globular conformation, and the deprotonated peptides formed intermolecular hydrogen bonds between the copolymer chains. This pH- and thermo-induced conformational transition resulted in the formation of large aggregates. These results imply that the morphology of this grafted copolymer can be controlled by multiple stimuli.
Similar content being viewed by others
Introduction
In the last decade, various fields have focused considerable attention on stimuli-responsive polymers.1 These polymers can be used in drug delivery, tissue engineering, biosensing and separation processes because of their sensitivity to environmental changes.2, 3, 4 There are many possible stimuli, such as temperature, pH, light and electric fields; most intensive investigations have focused on temperature- and pH-responsive polymers for biomedical applications.5, 6 To date, most dual thermo- and pH-sensitive polymers are prepared by incorporating pH-responsive ionic components such as carboxyl and amino groups into thermosensitive polymers. Thermosensitive polymers exhibit a lower critical solution temperature in aqueous solution, below which the polymers are water soluble and above which they become insoluble. Poly(N-isopropylacrylamide) (PNIPAm) and its copolymers are the most extensively studied lower critical solution temperature-type thermosensitive polymers. Polymers exhibiting lower critical solution temperature properties have potential applications for ‘intelligent’ or ‘smart’ materials. The thermoresponsive nature of these polymers has led to applications in drug delivery,7 bioengineering8 and nanotechnology9 and suggests a promising future for applications in the areas of biosensors and membranes.
Helices and β-sheets are the major secondary structural motifs organizing the three-dimensional geometry of proteins. The conformational and morphological changes of proteins and peptides induced by chemical and/or physical stimuli have attracted attention not only because of the functional regulation owing to their characteristic structure10 but also because of their association with neurodegenerative diseases11 such as Alzheimer’s and Creutzfeldt-Jacob’s. In previous studies,12, 13, 14 we reported that a simple amphiphilic copolymer, hydrophilic peptide-grafted polyallylamine, formed amyloid-like fibrils in aqueous solution under acidic conditions. The pH-induced reversible conformational transition was also observed.15 Studies on the structural regulation for peptides forming a β-sheet may be important not only for understanding the pathogenesis and therapeutics of certain diseases but also for providing useful information for the development of nanobiomaterials with a wide range of applications, such as in nanodevices.
Peptide-conjugated PNIPAm systems have been widely studied.16, 17 Mezzenga and co-workers18 used poly(N-isopropylamide) conjugated with peptide to form biocompatible hydrogels, but these polymers were only responsive to single stimuli. In this paper, we describe the pH- and thermo-induced conformational and morphological changes of (leucine-lysine)8-grafted PNIPAm in aqueous solution. In combining the pH-sensitive PNIPAm (Leu-Lys)8 with the temperature-responsive PNIPAm, we demonstrated the creation of a multi-stimuli-responsive polymer system. Under acidic conditions, the amino group of the Lys moieties of the (Leu-Lys)8 graft chain was protonated, and the electrostatic repulsion disturbed the formation of the β-sheet structure. However, under basic conditions, the deprotonated (Leu-Lys)8 graft chain could form β-sheets via intermolecular hydrogen bonding. However, below the coil-to-globule transition temperature of the main chain, the intermolecular hydrogen bonding among the (Leu-Lys)8 graft chains was disturbed owing to the coil conformation. The (Leu-Lys)8 graft chain took a stable β-sheet structure only under basic conditions and above the transition temperature. The β-sheet conformation induced the formation of large aggregates of the peptide-grafted copolymers. This multi-stimuli-responsive polymer system could be useful for various applications, such as drug delivery and membrane separation.
Experimental procedure
Materials
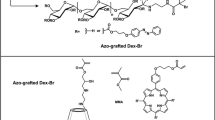
The pH- and thermo-responsive graft copolymer (Leu-Lys)8-grafted PNIPAm (LKNIPAm) was prepared as shown in Scheme 1. The sequence (Leu-Lys)8 was chosen as a pH-dependent, β-sheet-forming element. It is well known that sequential alternating amphiphilic peptides take a β-sheet conformation, and pH-induced conformational transitions of peptides have been reported.19
First, the peptide chain (Leu-Lys)8-vinyl was synthesized with a vinyl group at the N-terminal by means of the conventional solid-phase method.20 The peptide chain (Leu-Lys)8 was synthesized on a CLEAR-acid resin (cross-linked ethoxylate acrylated resin, Peptide Institute, Osaka, Japan), using Fmoc-amino-acid derivatives (3 equiv), 1-hydroxy-7-azabenzotriazole (3 equiv) and 1,3-diisopropylcarbodiimide (3 equiv) in N,N-dimethylformamide for coupling and using piperidine (25vol%)/N,N-dimethylformamide to remove the Fmoc. The acrylic acid was then attached to the N-terminal of the peptide on the resin using the same protocol described above. After the coupling reactions, to cleave the (Leu-Lys)8-vinyl from the resin and remove the side-chain-protecting groups, the peptide resin was treated with a cooled aqueous solution of 95 vol% trifluoroacetic acid. After the reaction, the mixture was filtered to separate the peptide solution from the resin support. The trifluoroacetic acid solution of the peptide was concentrated to a volume of approximately 1–2 ml, and then 100 ml of cooled ether was added to precipitate the peptide. The peptide was identified by MALDI-TOF mass spectroscopy (JMS-S3000, JEOL, Tokyo, Japan). The observed m/z of the peptide was 2023.9. This value was in fair agreement with the calculated value of 2024.7 [M+Na]+.
We chose PNIPAm as the thermo-responsive hydrophilic main chain of the grafted copolymer. (Leu-Lys)8-vinyl (20 mg) and NIPAm (4.52 mg) were dissolved in aqueous solution. To remove the oxygen from the reaction mixture, three freeze–pump–thaw cycles were performed. The copolymerization of the (Leu-Lys)8-vinyl and NIPAm was initiated by azodiisobutyronitrile (AIBN) through a conventional radical polymerization method. The AIBN (0.2 mg) was added to the reaction mixture, which was then stirred for 48 h at 70 °C. The reaction mixture was then precipitated into excess ether. The precipitate was dissolved in water, and the aqueous solution was dialyzed against water using a molecular porous membrane tube (BioDesign Inc., Carmel, NY, USA, MWCO 3500). After the dialysis, the solution was lyophilized to obtain LKNIPAm. The graft peptide content of 12 mol% in LKNIPAm was estimated by means of 1H-NMR spectroscopy in deuterated trifluoroacetic acid, on the basis of the area ratio of the signal of –NH–CH–CO– (δ=4.1 p.p.m.) of the peptide graft chain to that of –CH3 (δ=1.3 p.p.m.) of the methyl groups of the NIPAm and Leu side chains. The molecular weight of the obtained LKNIPAm was estimated by size-exclusion chromatography, calibrated with polystyrene standards using a pump system of Tosoh DP8020 with a TSK-GEL α-3000 column (eluent; N,N-dimethylformamide, flow rate; 0.5 ml min−1, temperature; 40 °C). The number-average molecular weight of LKNIPAm was 1.3 × 104 (Mw/Mn was 2.7).
Spectroscopic measurements
The pH-induced conformational changes of (Leu-Lys)8-vinyl and the peptide graft chain of the LKNIPAm in aqueous solution were investigated by means of circular dichroism (CD). CD spectra were recorded on a J-820 spectrophotometer (JASCO, Tokyo, Japan) under a nitrogen atmosphere. Experiments were performed in a quartz cell with 0.1-cm path length from 190–250 nm at ambient temperature. The pH of the solution was adjusted with 0.1 M HCl or 0.1 M NaOH.
The thermo-induced changes in the secondary structure of the peptide graft chain in LKNIPAm were estimated by transmittance Fourier transform infrared spectroscopy. The transmittance Fourier transform infrared spectra were measured with a Perkin-Elmer Spectra 2000 (reduction: 4 cm−1, number of scans: 32, Perkin-Elmer, Waltham, MA, USA). The LKNIPAm aqueous solutions at various pH concentrations and temperatures were quickly frozen in liquid nitrogen, and then the frozen samples were lyophilized to obtain LKNIPAm powder. The pellets for transmittance Fourier transform infrared measurements were prepared by mixing the LKNIPAm powder with KBr. The weight fraction of the LKNIPAm was fixed at 1 wt%.
The thermo-induced turbidity changes of the LKNIPAm aqueous solution were investigated by means of their transmittance changes. The transmittance at 450 nm of the LKNIPAm aqueous solutions was measured with the J-820 spectrophotometer equipped with a temperature control accessory (PTC-423L, JASCO). The transmittance values at 450 nm were obtained by the conversion of the high-tension voltage at 450 nm.
Transmission electron microscopy
The morphology of LKNIPAm in aqueous solution at various pH concentrations and temperatures was directly observed by transmission electron microscopy using a freeze–fracture–etching replica technique.21, 22 An aliquot of the LKNIPAm solution at the designated pH and temperature was placed on a thin gold plate, and this sample was rapidly plunged into liquid nitrogen (EM-19510SNPD, JEOL). The sample was stored in liquid nitrogen until it fractured. Freeze fracturing was carried out with a freeze-etching system (JFD-II, JEOL) at −170 °C and 3.4 × 10−5 Pa, and then freeze-etching was performed at −120 °C and 3.5 × 10−5 Pa for 15 min. To prepare the replica, platinum-carbon and pure carbon were evaporated at angles of 60° and 90°, respectively, to the specimen surface. Electron microscopy was carried out using a JEOL z2500 electron microscope operating at 200 kV.
Results and discussion
pH-induced conformational changes of (Leu-Lys)8
The secondary structure and morphology of β-sheet peptides have been widely studied. Sequential alternating amphiphilic peptides composed of hydrophobic and hydrophilic amino acids have been shown to take a β-sheet structure and form fibrous assemblies under specific conditions, such as a certain pH and/or solvent composition.19, 23, 24, 25 The pH-induced conformational changes of (Leu-Lys)8-vinyl were characterized by CD. The concentration of (Leu-Lys)8-vinyl was fixed at 0.8 mM. Figure 1 shows the pH-induced CD spectral changes of (Leu-Lys)8-vinyl in aqueous solution when the pH of the solution was increased (Figure 1a) and decreased (Figure 1b). Under acidic conditions, (Leu-Lys)8-vinyl showed a negative maximum at 198 nm, indicating a random coil conformation. Under basic conditions, the CD spectra of (Leu-Lys)8-vinyl changed to a typical β-sheet pattern, which shows a negative maximum at 215 nm. We obtained the fraction of the second-order structure using a quantitative curve-fitting analysis of the CD spectrum according to a linear combination of typical CD spectra for dispersed α-helical, β-sheet and random coil conformations.26 The turbidity of the peptide solution increased slightly under basic conditions, owing to the formation of the β-sheet assembly. The transmittance at 450 nm at pH 9.5 was 97.9%; this value was slightly lower than the value at pH 3.0 (98.8%).
Figure 2 shows the pH-induced conformational changes of (Leu-Lys)8-vinyl in aqueous solution. Reversible transitions between the random coil and β-sheet conformations were observed. However, a considerable hysteresis was observed in the pH-induced transition.
pH dependence of the fraction of the second-order structure.  ,
,  α-helix,
α-helix,  ,
,  : β-sheet, and
: β-sheet, and  ,
,  : random coil conformation of (Leu-Lys)8-vinyl estimated from the circular dichroism-curve-fitting method. The open and closed symbols denote the fraction of the second-order structure when the pH of the solution was increased and decreased, respectively.
: random coil conformation of (Leu-Lys)8-vinyl estimated from the circular dichroism-curve-fitting method. The open and closed symbols denote the fraction of the second-order structure when the pH of the solution was increased and decreased, respectively.
This pH-induced random coil/β-sheet conformational transition behavior arises from the difference in the ionization of the side chain in the (Leu-Lys)8-vinyl. Under basic conditions, (Leu-Lys)8-vinyl formed a stable β-sheet through intermolecular hydrogen bonding. Meanwhile, under acidic conditions, the amino group of the Lys side chain was positively charged. The electrostatic repulsion among the protonated amino groups of the Lys moieties disturbs the intermolecular hydrogen bonding, interrupting β-sheet formation and leading to random coil conformation.
We compared the pH-induced conformational transition of the peptide graft chain in the LKNIPAm with that of the free peptide (Leu-Lys)8-vinyl. Figure 3 shows the pH-induced CD spectral changes of LKNIPAm in aqueous solution. The CD measurements, as the pH of the solution was increased (Figure 3a) and decreased (Figure 3b), were carried out immediately after adjustment of the pH of the solution at ambient temperature. The concentration of the peptide graft chain was fixed at 0.03 mM. All the CD spectra of LKNIPAm showed a negative maximum at approximately 218 nm, which indicates the existence of a β-sheet structure. As the graft peptide was fixed on the PNIPAm main chain, the local concentration of the graft peptide around the PNIPAm main chain was increased in comparison with the same concentration of the free peptide (Leu-Lys)8-vinyl. At equal concentrations of peptide (0.03 mM), [θ]218 nm of LKNIPAm was larger than that of the free peptide (Leu-Lys)8-vinyl (Supplementary Figure S1), indicating that the (Leu-Lys)8 graft chain of LKNIPAm could more easily adopt a β-sheet structure compared with the free peptide (Leu-Lys)8-vinyl, owing to the increased local concentration. In other words, increasing the local concentration of the graft peptide induces intermolecular hydrogen bonding and the formation of a β-sheet structure. The CD spectra of LKNIPAm could not be fitted by a linear combination of typical CD spectra for dispersed α-helix, β-sheet and random coil conformations. Figure 4 shows the pH dependence of the molar ellipticity at 218 nm, [θ]218 nm, assigned to the β-sheet structure for LKNIPAm in aqueous solution, as the pH of the solution was increased (○) or decreased (•). A large hysteresis was observed in the pH-induced conformational transition of the (Leu-Lys)8 graft chains in LKNIPAm. This result implies that the rate of the conformational transition from stable β-sheet to random coil was slower than that of random coil to β-sheet, when compared with the case of the free (Leu-Lys)8-vinyl.
pH- and thermo-induced conformational changes of LKNIPAm
The conformation of (Leu-Lys)8-vinyl did not change with temperature (from 10–45 °C) under acidic or basic conditions. In this research, we chose PNIPAm27, 28 as the thermoresponsive part of the multi-stimuli-responsive copolymer. We investigated the thermo-induced turbidity changes owing to the coil-to-globule transition of the main chain in LKNIPAm by the transmittance at 450 nm in aqueous solution. Figure 5 shows the thermo-induced transmittance changes at 450 nm of the LKNIPAm aqueous solution at pH 3.0 (Figure 5a) and pH 9.0 (Figure 5b). At pH 3.0, the transmittance at 450 nm did not change and remained relatively high. However, at pH 9.0, the transmittance drastically increased with increasing temperature, beginning at approximately 37 °C. In this case, LKNIPAm partially precipitated above 40 °C. We also investigated the thermo-induced conformational changes of the (Leu-Lys)8 graft chain in LKNIPAm at 25 °C and 40 °C by Fourier transform infrared measurements. The samples for the Fourier transform infrared measurements were prepared as follows. The pH and temperature of the LKNIPAm aqueous solution were adjusted to the desired values (pH 3.0, pH 9.0, 25 °C, 40 °C). The LKNIPAm solutions were quickly frozen in liquid nitrogen and lyophilized to obtain measurement samples. We had earlier verified that no conformational changes had occurred during the lyophilization process. The secondary structure of the water-soluble peptide in aqueous solution was measured by CD. The fraction of the second-order structure obtained by the Fourier transform infrared measurement of the lyophilized peptide was in fair agreement with that obtained by the CD measurement in aqueous solution. Figure 6 shows the pH- and thermo-induced Fourier transform infrared spectra of LKNIPAm. In the spectra, characteristic absorptions of the amide I band in α-helix, β-sheet and random coil conformations were observed at 1650, 1630 and 1675 cm−1, respectively.29 The ratio of the integrated peak intensities assigned to the individual secondary structures, which was obtained by peak deconvolution of the amide I band, gave the percentage of the different conformations of the (Leu-Lys)8 graft chain in LKNIPAm. The results of the conformational analysis are summarized in Table 1. Stable β-sheet structure formation among the (Leu-Lys)8 graft chains occurred only under basic conditions at high temperature (pH 9.0 and 40 °C). The observed pH- and thermo-induced conformational transition of LKNIPAm in aqueous solution could be explained as follows. Under acidic conditions (pH 3.0), the (Leu-Lys)8 graft chain of LKNIPAm was protonated and positively charged. The ionized graft peptides increased the water solubility of LKNIPAm at high temperatures, at which the PNIPAm main chain forms a globular conformation. Meanwhile, under basic conditions (pH 9.0), the (Leu-Lys)8 graft peptides were neutral. At low temperatures, the PNIPAm main chain forms a coil conformation. The conformation of the main chain of LKNIPAm disturbed the β-sheet formation of the peptide graft chain. The hydrophobic interactions among the peptide graft chains resulted in the formation of a micellar structure. The formation of these LKNIPAm micelles induced the decrease in transmittance at 450 nm. At high temperature (40 °C), the PNIPAm main chain formed a globular conformation. Under these conditions, the grafted peptide chains formed a β-sheet conformation. The β-sheet structure of the peptide graft chains acted as bridging points among the LKNIPAms micelles, resulting in precipitation.
Fourier transform infrared spectra of LKNIPAm prepared by quick freezing and lyophilization at (a) pH 3.0 and 25 °C, (b) pH 3.0 and 40 °C, (c) pH 9.0 and 25 °C and (d) pH 9.0 and 40 °C. Broken lines show the peak deconvolution of the amide I band due to (1) α-helix, (2) β-sheet and (3) random coil conformations.
pH- and thermo-induced morphological changes of LKNIPAm in aqueous solution
The pH- and thermo-induced morphological changes of LKNIPAm owing to the conformational transitions of the main chain (PNIPAm) and peptide graft chains (Leu-Lys)8 in aqueous solution were observed directly with a transmission electron microscope using a freeze–fracture–etching replica technique. The LKNIPAm aqueous solution at the desired pH and temperature was quickly frozen, and the replicas were prepared by the freeze–fracture–etching technique. Figure 7 shows the transmission electrone microscope images of LKNIPAm in aqueous solution. Under acidic conditions (pH 3.0), the (Leu-Lys)8 graft chain of LKNIPAm was positively charged and took a random coil and α-helical conformation with a considerable amount of β-sheet structure (Figures 6a and b). At the low temperature of 25 °C, below the coil-to-globule transition temperature, the main chain of LKNIPAm formed a coil conformation. Under these conditions, LKNIPAm formed water-soluble nanoparticles, in which the partially formed β-sheet structure of the grafted peptides bridged the main chains of LKNIPAm. In Figure 7a, nanoparticles with a diameter of 3–45 nm were observed. By increasing the temperature to 40 °C, above the coil-to-globule transition temperature, the PNIPAm main chain adopted a shrunken globule form. However, the (Leu-Lys)8 graft chains of the LKNIPAm were charged, and the formation of the β-sheet structure was disturbed by the electrostatic repulsion between the graft chains. The morphology of LKNIPAm in this case was a maintained dispersed particle with a diameter of 10–60 nm (Figure 7b). In basic solution (pH 9.0) and at low temperature (25 °C), LKNIPAm formed relatively larger particles compared with those formed under acidic conditions (Figure 7c, diameter 30–150 nm). In this case, the PNIPAm main chain took on a water-soluble coil conformation, and the peptide graft chains were neutral. However, the formation of the β-sheet structure that acted as a bridging point between the LKNIPAm polymer strands was disturbed by the expanded flexible coil conformation of the PNIPAm main chain. These effects resulted in the formation of relatively large dispersed particles. At the higher temperature of 40 °C, PNIPAm adopted a shrunken globule conformation, and the (Leu-Lys)8 graft chains formed a stable β-sheet structure (Figure 6d). Figure 7d shows the LKNIPAm morphology at pH 9.0 and 40 °C in aqueous solution. In this image, a large aggregate can be observed. This aggregated body was formed by the cross-linking of LKNIPAm, whose main chain was a shrunken globule, through the formation of bridging β-sheets of the grafted peptide chains.
Conclusions
In conclusion, the amphiphilic copolymer (Leu-Lys)8-grafted PNIPAm shows pH- and thermo-induced conformational and morphological transitions in aqueous solution. Schematic pictures of the proposed pH- and thermo-induced structural changes of LKNIPAm are illustrated in Scheme 2. Under acidic conditions, the peptide graft chains were protonated and positively charged, and LKNIPAm formed nanoparticles owing to the electrostatic repulsion among the graft chains above and below the coil-to-globule transition temperature of the PNIPAm main chain. However, under basic conditions, the peptide graft chains were neutral, and, at a low temperature below the transition temperature of the main chain, LKNIPAm formed a micellar structure. Only at a temperature higher than the transition temperature, in which the main chain formed shrunken globules, did the peptide graft chains form a stable β-sheet structure. The β-sheets of the grafted chains acted as bridging points between the LKNIPAm globules, resulting in the formation of a large aggregated body.
We believe that these studies on the structural regulation of multi-stimuli-responsive polymers provide useful information for the development of novel stimuli-responsive materials with a wide range of applications in nanotechnology.

Synthetic route of the amphiphilic peptide-grafted copolymer, LKNIPAm.

Schematic of the pH- and thermo-induced structural changes of LKNIPAm in aqueous solution.
References
Gil, E. S. & Hudson, S. M. Stimuli-responsive polymers and their bioconjugates. Prog. Polym. Sci. 29, 1173–1222 (2004).
Bae, Y., Fukushima, S., Harada, A. & Kataoka, K. Design of environment-sensitive supramolecular assemblies for intracellular drug delivery: polymeric micelles that are responsive to intracellular pH change. Angew. Chem. Int. Ed. 42, 4640–4643 (2003).
Nitschke, M., Gramm, S., Götze, T., Valtink, M., Drichel, J., Voit, B., Engelmann, K. & Werner, C. Thermo-responsive poly(NiPAAm-co-DEGMA) substrates for gentle harvest of human corneal endothelial cell sheets. J. Biomed. Mater. Res. Part A. 80A, 1003–1010 (2007).
Reppy, M. A. & Pindzola, B. A. Biosensing with polydiacetylene materials: structures, optical properties and applications. Chem. Commun. 42, 4317–4338 (2007).
Tachibana, Y., Kurisawa, M., Uyama, H. & Kobayashi, S. Thermo- and pH-responsive biodegradable poly(α-N-substituted γ-glutamine)s. Biomacromolecules 4, 1132–1134 (2003).
Cai, Y., Tang, Y. & Armes, S. P. direct synthesis and stimulus-responsive micellization of Y-shaped hydrophilic block copolymers. Macromolecules 37, 9728–9737 (2004).
Eeckman, F., Moës, A. J. & Amighi, K. Synthesis and characterization of thermosensitive copolymers for oral controlled drug delivery. Eur Polym J 40, 873–881 (2004).
Pennadam, S. S., Lavigne, M. D., Dutta, C. F., Firman, K., Mernagh, D., Górecki, D. C. & Alexander, C. Control of a multisubunit DNA motor by a thermoresponsive polymer switch. J. Am. Chem. Soc. 126, 13208–13209 (2004).
Ito, T., Hioki, T., Yamaguchi, T., Shinbo, T., Nakao, S. I. & Kimura, S. Development of a molecular recognition ion gating membrane and estimation of its pore size control. J. Am. Chem. Soc. 124, 7840–7846 (2002).
Janek, K., Behlke, J., Zipper, J., Fabian, H., Georgalis, Y., Beyermann, M., Bienert, M. & Krause, E. Water-soluble β-sheet models which self-assemble into fibrillar structures. Biochemistry. 38, 8246–8252 (1999).
Lansbury, P. T. A reductionist view of Alzheimer's disease. Acc. Chem. Res. 29, 317–321 (1996).
Koga, T., Taguchi, K., Kinoshita, T. & Higuchi, M. pH-regulated formation of amyloid-like β-sheet assemblies from polyglutamate grafted polyallylamine. Chem. Commun. 3, 242–243 (2002).
Koga, T., Taguchi, K., Kobuke, Y., Kinoshita, T. & Higuchi, M. Structural regulation of a peptide-conjugated graft copolymer: a simple model for amyloid formation. Chem. Eur. J. 9, 1146–1156 (2003).
Koga, T., Taguchi, K., Kogiso, M., Kobuke, Y., Kinoshita, T. & Higuchi, M. Amyloid formation of native folded protein induced by peptide-based graft copolymer. FEBS Lett. 531, 137–140 (2002).
Higuchi, M., Inoue, T., Miyashi, H. & Kawaguchi, M. pH-induced reversible conformational and morphological regulation of polyleucine grafted polyallylamine assembly in solution. Langmuir. 21, 11462–11467 (2005).
Trzebicka, B., Robak, B., Trzcinska, R., Szweda, D., Suder, P., Silberring, J. & Dworak, A. Thermosensitive PNIPAM-peptide conjugate—synthesis and aggregation. Eur. Polym. J. 49, 499–509 (2013).
Turk, M. J., Reddy, J. A., Chmielewski, J. A. & Low, P. S. Characterization of a novel pH-sensitive peptide that enhances drug release from folate-targeted liposomes at endosomal pHs. Biochim. Biophys. Acta 1559, 56–68 (2002).
Li, C., Mohammad, M. A., Bolisetty, S., Adamcik, J. & Mezzenga, R. New biocompatible thermo-reversible hydrogels from PNIPAm-decorated amyloid fibrils. Chem. Commun. 47, 2913–2915 (2011).
Hong, D. P., Hoshino, M., Kuboi, R. & Goto, Y. Clustering of fluorine-substituted alcohols as a factor responsible for their marked effects on proteins and peptides. J. Am. Chem. Soc. 121, 8427–8433 (1999).
Carpino, L. A. & Han, G. Y. 9-fluorenylmethoxycarbonyl amino-protecting group. J. Org. Chem. 37, 3404–3409 (1972).
Fassel, T. A., Sek-wen, H., Leonards, K. & Ohki, S. Electron microscopic study of the calcium phosphate-induced aggregation and membrane destabilization of cytoskeleton-free erythrocyte vesicles. Biochim. Biophys. Acta 943, 267–276 (1988).
Higuchi, M., Kinoshita, T., Takizawa, A., Tsujita, Y. & Okochi, K. Channel forming activity of an anionic amphiphilic sequential polypeptide in a cationic bilayer membrane. Bull. Chem. Soc. Jpn. 63, 1916–1920 (1990).
Fukushima, Y. Alcohol-induced helix-sheet transition of a sequential alternating amphiphilic polypeptide. Chem. Lett 28, 157–158 (1999).
Sugimoto, N., Zou, J., Kazuta, H. & Miyoshi, D. Structural transition of short oligopeptides by water/organic solvent titration. Chem. Lett. 28, 637–638 (1999).
Koga, T., Matsuoka, M. & Higashi, N. Structural control of self-assembled nanofibers by artificial β-sheet peptides composed of d- or l-Isomer. J. Am. Chem. Soc. 127, 17596–17597 (2005).
Greenfield, N. J. & Fasman, G. D. Computed circular dichroism spectra for the evaluation of protein conformation. Biochemistry. 8, 4108–4116 (1969).
Christine, M. S., Zhang, M., Rizzardo, E., Thang, S. H., Chong, Y. K., Edwards, K., Karlsson, G. & Muller, A. H. E. A new double-responsive block copolymer synthesized via raft polymerization: Poly(N-isopropylacrylamide)-block-poly(acrylic acid). Macromolecules 37, 7861–7866 (2004).
Lee, R. S., Chen, W. H. & Huang, Y. T. Synthesis and characterization of dual stimuli-responsive block copolymers based on poly(N-isopropylacrylamide)-b-poly(pseudoamino acid). Polymer 51, 5942–5951 (2010).
Miyazawa, T. & Blout, E. R. The Infrared spectra of polypeptides in various conformations: amide I and II Bands. J. Am. Chem. Soc. 83, 712–719 (1961).
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on Polymer Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Yu, M., Tang, T., Takasu, A. et al. pH- and thermo-induced morphological changes of an amphiphilic peptide-grafted copolymer in solution. Polym J 46, 52–58 (2014). https://doi.org/10.1038/pj.2013.61
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pj.2013.61







 ) and decreased (
) and decreased ( ).
).

