Abstract
A triblock terpolymer, polystyrene-block-polybutadiene-block-poly(methyl methacrylate) (SBM), formed a double-helical nanoscale structure, composed of polybutadiene (PB) helical microdomains around hexagonally packed polystyrene (PS) cores in a poly(methyl methacrylate) matrix. The orientation of double-helical morphologies at various film thicknesses were studied using transmission electron microtomography, following solvent annealing and drying at a controlled solvent evaporation rate. The evaporation rate of the solvent and the film thickness were important factors in whether the double-helical microdomains were oriented parallel or perpendicular with respect to the substrate. In some cases, the perpendicularly aligned double-helical morphology extended several micrometers from the substrate to the air surface. A similar experiment using polystyrene-block-poly(methyl methacrylate) diblock copolymer proved that the presence of helical PB microdomains around PS cylinders is the key factor in achieving a uniform orientation over several micrometers throughout the film thickness.
Similar content being viewed by others
Introduction
Microphase-separated structures of block copolymers have attracted intense interest as building blocks for mesoscale (tens of nanometers) structures formed by self-assembly processes. Microphase-separated structures have been used as membranes, scaffolds and as nanotemplates for lithographic applications or nanoporous materials for the next generation of electronic and photonic materials.1, 2 In order to fabricate structures for these applications, the orientation of the microphase-separated structure is a key issue. Asymmetric diblock copolymers, which form a hexagonally packed cylindrical structure, tend to align parallel to the surface because of the preferential interaction of one of the constituent blocks with the substrate. Many techniques have been used to orient the cylindrical structures perpendicular to the substrate in the copolymer film, including surface affinity,3, 4, 5, 6 the application of shear force,7 electric fields,8, 9, 10, 11 and magnetic fields.12, 13, 14, 15, 16 In addition, surface roughness is critical for a vertical orientation.17 However, few of these techniques can maintain the perpendicular orientation in the copolymer thick film over several micrometers. It has been theoretically and experimentally found that the distance that the surface-directed perpendicular orientation propagates is limited to roughly one period of the microphase-separated structure.5, 18, 19 Some studies have also shown that the orientation of microphase-separated structures can be controlled by exposing block copolymer films to solvent vapor and drying at a controlled evaporation rate.13, 18, 20, 21, 22, 23, 24, 25, 26, 27
We have reported the experimental visualization of a three-dimensional (3D) helical morphology for a polystyrene-block-polybutadiene-block-poly(methyl methacrylate) triblock terpolymer (SBM) using transmission electron microtomography (TEMT).28 The TEMT observations revealed that the double-helical structure was composed of polybutadiene (PB) helical microdomains around hexagonal-packed polystyrene (PS) cylinder cores in a poly(methyl methacrylate) (PMMA) matrix, even though the block copolymer was not chiral. This helical structure may have great potential as soft materials for new applications, such as templates for fabricating nanosprings or nanocoils. As with the cylinder-forming triblock copolymer system, techniques for controlling the orientation of the double-helical morphology are not well established.
In this paper, the orientation of the double-helical morphology in the SBM films is controlled by applying a solvent evaporation method, which consists of swelling the film with a solvent vapor and then drying it at a controlled evaporation rate. Structural observation of the films using transmission electron microscopy (TEM) and TEMT revealed that the orientation of the morphologies depends on the film thickness and the solvent evaporation rate. Furthermore, the SBM morphologies show persistent lengths of several micrometers, which are much longer than those of the cylinder-forming diblock copolymer systems. This indicates that the lower affinity for the solvent of the PB helical microdomain around the PS cylindrical core is a key factor in stabilizing the structural orientation against solvent perturbation during evaporation.
Experimental Procedure
Materials
The synthesis of the SBM triblock terpolymer has been described elsewhere.28 The terpolymer possesses a number-average molecular weight (Mn) of 170 kg mol−1 with a polydispersity index (Mw/Mn) of 1.06. Polystyrene-block-poly(methyl methacrylate) (SM) diblock copolymer (Mn=205 kg mol−1 and Mw/Mn=1.03) was purchased from Polymer Source Inc. (Dorval, Quebec, Canada). Further characteristics of the two block copolymers are given in Table 1.
Sample preparation and solvent annealing
The SBM triblock terpolymer films and the SM diblock copolymer films were prepared by spin-coating chloroform (CHCl3) solutions onto a silicon wafer at 4000, r.p.m. The film thickness (h) was controlled by varying the concentration from 0.4 to 8 wt%. Before use, the silicon wafer was cleaned with piranha solution (H2SO4/H2O2=3:1) at 80 °C for 20 min, then rinsed in deionized water several times and dried with N2 gas. Immediately after spin casting, the as-spun films were placed in a 1.2 l sample chamber that was filled with saturated CHCl3 vapor (Figure 1) for 2 days at 25 °C (solvent annealing). After solvent annealing, N2 gas was blown through the sample chamber at a constant flow rate. The evaporation rate of chloroform from the films was thus controlled by the N2 flow rate (R), which was between 20 and 80 ml min−1.
The polymer films were removed from the chamber after they had dried completely, and were coated with a carbon layer (∼10 nm) using a vacuum evaporator (JEOL JEE-420 T, JEOL Co., Ltd., Tokyo, Japan). Subsequently, the films were floated off the Si wafer using acidic NH4F solution. Some films were transferred to Cu mesh grids with a polyvinyl formal supporting film, and others were embedded in an acrylic resin for sectioning by ultramicrotome (Leica Ultracut UCT, Leica Microsystems GmbH, Wetzlar, Germany) at room temperature with diamond knives, perpendicular to the film surface in order to observe the cross-section. The ultrathin sections were transferred onto the Cu mesh grids. Before the TEM and 3D TEMT observations, the sections on the grids were exposed to OsO4 vapor for several hours to reveal the PB domain in SBM films, or to RuO4 vapor for several minutes to reveal the PS domain in the SBM or SM films. Finally, gold particle (∼10 nm diameter) dispersions were dropped onto the grids.
TEM and 3D TEMT observations
The TEM and 3D TEMT observations were carried out on a JEM-2200FS (JEOL Co., Ltd.) operated at 200 kV and equipped with a slow-scan CCD camera (Gatan USC 4000, Gatan Inc., Pleasanton, CA, USA). A series of TEM images were acquired at tilt angles from −75 ° to +75 ° at an angular interval of 1 °. The tilt series of the TEM images were aligned by the fiducial marker method using the gold nanoparticles as the fiducial markers and then reconstructed with the filtered-back-projection algorithm.29, 30
Results and Discussion
Morphology and microdomain orientation
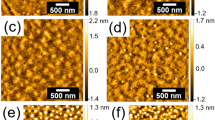
To study the effect of the solvent evaporation rate on the orientation of microphase-separated structures, SBM thin films were prepared by using a wide range of solvent evaporation rates. Figure 2a and d shows the morphologies in the as-spun SBM thin films and those after solvent annealing. The dark domains correspond to the PB microdomains, which were selectively stained using OsO4. Figure 2a shows that the morphology in the as-spun film is disordered with no distinct periodicity. After the solvent annealing and controlled solvent evaporation, the double-helical structure, composed of PB helical microdomains around hexagonal-packed PS cylinder cores in a PMMA matrix, was found in the SBM films (Figure 2b and d). The diameter of the cylindrical PS microdomains is ∼25 nm, and the diameter and helical pitch of the PB microdomains were ∼10 nm and ∼45 nm, respectively (Figure 2f). The morphological change from the disordered to the double-helical structure suggests that the microphase-separated structure developed during the solvent annealing and evaporation process. More importantly, the orientation of the double-helical structures varied dramatically, depending on the N2 flow rate. A highly ordered morphology oriented parallel to the substrate was observed at R=20 ml min−1 (Figure 2b). In contrast, a highly ordered morphology with a perpendicular orientation was observed at R=50 ml min−1 (Figure 2c). Figure 2c also shows the well-defined lateral ordering of the double-helical structure, which is nearly defect-free over an area of 1.5 × 1.5 μm2. However, as R increased, the main orientation reverted to parallel at R=80 ml min−1 (Figure 2d). When the sample chamber was opened immediately after solvent annealing, the microdomains appeared highly disordered (Figure 2e), which was similar to the as-spun films. The helical structures, which had already formed during the solvent annealing, were disordered because of the fast N2 gas flow in the drying processes (discussed later in relation to Table 2).
TEM micrographs of the SBM thin film (∼300 nm thick). Top view: (a) Morphology of the as-spun film. Morphology observed after solvent annealing for 2 days, at an N2 flow rate of (b) 20 ml min−1, (c) 40 ml min−1, (d) 60 ml min−1 and (e) after the sample chamber was opened directly after solvent annealing. (f) Schematic illustration of the microphase-separated structure in the SBM film.
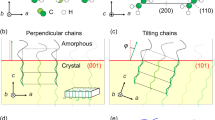
In addition, the orientation varied as a function of the film thickness (h). For a thinner film, where h=190 nm, the TEM cross-sectional view showed a parallel orientation (Figure 3a), then as h increased to 380 nm, a perpendicular morphology was observed (Figure 3b). Moreover, as h increased to 5 μm (Figure 3c), the perpendicular morphology was formed only near the air and substrate surfaces (Figure 3d and f), and in the central region of the film, the helical microdomains were inclined and disordered (Figure 3e).
TEM cross-sectional views of the SBM thin films after solvent annealing at an N2 flow rate of 60 ml min−1. Film thicknesses: (a) 190 nm, (b) 380 nm and (c) 5 μm. Magnified images of the areas indicated in (c): (d) near the free surface, (e) in the central region and (f) near the substrate interface.
As the N2 flow rate and the film thickness strongly affected the orientation of microdomains in the SBM films, an orientation diagram was constructed by plotting the domain orientation as a function of h vs R (Figure 4).
Orientation diagram of SBM thin films as a function of the film thickness and the flow rate of N2 (h–R diagram). The dashed line indicates a transformation between the parallel and perpendicular orientations, and the dashed circle indicates the disturbed perpendicular orientation shown in Figure 3c.
When h was <100 nm, the orientation of the helical microdomains was parallel to the surface regardless of R. As h increased, the perpendicular orientation emerged when R was around 50 ml min−1. The perpendicular region grew larger as the thickness increased.
The interaction parameters, χ, between the three components in SBM are 0.03 (PS/PMMA), 0.045 (PS/PB) and 0.071 (PMMA/PB), as calculated by Stadler et al.,31 which indicate that the PB is highly immiscible with the other two components, and PS and PMMA are weakly immiscible with each other. Moreover, the solubility parameter δ values (Table 2) indicate that chloroform is a mutual solvent for PS and PMMA, but has lower affinity for PB. Thus, as the solvent concentration decreased during the evaporation at the free surface, the microphase separation that formed the PB microphase occurred. Meanwhile, the surface tension data in Table 2 indicate that the PB blocks prefer to be at the free surface and the substrate surface, because this results in the lowest surface free energy for the three components of the terpolymer. The PB helical domains are attached to both the substrate and the free surface in the parallel orientation, although the PB wetting layer cannot be formed on the substrate surface or the free surface because of the small fraction of PB blocks present (Figure 3a).
The parallel orientation would be dominant in the SBM films if the only factors were the preferential wetting of PB because of the surface tension and PB condensation during the solvent evaporation at the free surface. However, the solvent evaporation also seems to be a key factor. Russell et al.23 reported that highly oriented and ordered hexagonally packed cylindrical arrays were formed from PS-b-PEO block copolymers by solvent evaporation. Once the solvent evaporation starts, the solvent concentration is lowest at the free surface, and a concentration gradient is established normal to the film surface. As the solvent evaporates, the rearrangement of the softened helical structures begins, and the evaporation front propagates through the film, which produces a highly ordered and oriented array of cylindrical microdomains in the thin film.
In our experiments, the speed of this process is controlled by the N2 flow rate. When the flow rate is low (20 ml min−1), solvent evaporation is relatively slow, and the concentration gradient is likely to be shallow. Therefore, the existing orientation of the microphase-separated structure dominates. The dominant orientation of the helical structures is parallel in thinner films, and is directed by the effect of surface tension. A similar parallel orientation appeared at a high N2 flow rate (80 ml min−1). When the film dries within minutes, there is not enough time for the helical structures to change orientation, which leaves the existing parallel orientation unaltered. The helical morphology forms under the influence of surface attraction during the solvent annealing process.
The critical flow rate of N2 decreases for thinner films; the perpendicular orientation formed at a moderate N2 flow rate (40–60 ml min−1) when h≈300 nm, because of the effect of the solvent concentration gradient. In addition, the range of conditions where the perpendicular orientation forms increased as the film thickness increased. As the thickness increases to several micrometers, the solvent molecules have further to diffuse through the film from the substrate to the air surface, and the solvent flow will not remain laminar throughout the film thickness. An unstable vertical solvent flow would disturb the perpendicular orientation, which resulted in the inclined orientation observed in the middle of the thick films (Figure 3e).
Role of the PB microdomains
Figure 5 shows the highly homogenous orientation observed in the SBM film up to 1.1 μm in thickness. In this TEMT characterization, the direction along the helical structures was chosen as the tilting axis (Y axis) to reduce the information loss caused by the missing wedge phenomenon.28, 29 The TEMT characterization confirmed the presence of the double-helical structure after the orientation was altered by solvent annealing and evaporation. The corresponding 3D reconstructed images obtained by TEMT are overlaid in Figure 5a, and it is clear that the helical structures are oriented perpendicular to the surface of the polymer film. The slice in the X–Z plane (Figure 5b) shows well-defined hexagonal packing of the helical structures, which is highly homogenous over the whole volume that was observed (800 × 1200 × 450 nm3).
TEMT images of the SBM thick film (h=1.1 μm) with a perpendicular orientation controlled by solvent annealing and evaporation (R=50 ml min−1). Cross-sectional slice of the reconstruction in the X–Z plane (a) and in the X–Z plane (b). The corresponding 3D reconstructed images obtained by TEMT are overlaid on (a) with the PB microdomains colored blue and red. The tilt axis lies along the Y-direction and is indicated by the dashed line in (a). The dashed hexagon in (b) shows the hexagonal packing of the double-helical structures. The scale bar represents 200 nm and applies to both images.
An SM diblock copolymer with an S/M block ratio similar to that in the SBM triblock terpolymer was used to elucidate the role of the PB helical microdomain in orientation and ordering. The SM copolymer bulk morphology is hexagonally packed cylindrical PS microdomains in a PMMA matrix, which is similar to the bulk morphology of the SBM terpolymer, although it lacks the helical PB microdomains around the cylindrical PS cores. A typical SM thick film morphology, following solvent annealing and evaporation, is shown in Figure 6, where h=1.1 μm and R=50 ml min−1. The diameter of the cylindrical PS microdomain is ∼41 nm and the distance between the adjacent cylinders is ∼68 nm. The cylindrical PS microdomains cannot maintain the same orientation over the entire thickness of the SM film, and the persistence length of the PS cylinders is around 100 nm. In contrast, the morphology in the SBM film, generated under exactly the same experimental conditions, is a well-ordered perpendicular orientation (Figure 5). Figures 2, 3 and 5 show that the persistence length of the helical structure is extraordinarily long for both the perpendicular and the parallel orientation. In the thick film with a perpendicular orientation, the helical structure was nearly straight over the entire film thickness of several micrometers. The persistence length of the helical structures for the parallel orientation was usually several tens of micrometers.
The structural comparison of the SBM and the SM copolymers indicates that the middle block, the PB microdomain, has an important role in increasing the persistence length. During solvent annealing and evaporation, the solvent selectivity increases the solvent concentration in the PS and PMMA microdomains, whereas the solvent concentration in the PB microdomains is much lower. The solvent concentration and vertical flow during the evaporation process mean that the phase-separated structure begins to freeze from the free surface because of the low solvent concentration. However, the vertical solvent flow from the substrate surface to the free surface swells the helical structures near the free surface. As CHCl3 is a mutual solvent for both PS and PMMA, the cylindrical structure of the SM copolymer formed near the free surface is easily disturbed by the vertical solvent flow. In the SBM copolymer, the PB helical structure near the free surface is relatively stable to the vertical solvent flow because PB has low affinity for chloroform. The PB helices, which form ‘jacket tubes,’ stabilize the cylindrical PS microdomains against the vertical solvent flow.
Conclusions
Controlling the solvent evaporation rate of solvent-annealed SBM thin films up to several micrometers thick resulted in homogeneous parallel and perpendicular orientations of the double-helical microdomains. The well-defined and almost defect-free lateral ordering of the double-helical microdomain normal to the substrate was achieved over a 1.5 × 1.5 μm2 area. The perpendicular orientation of the helical morphology arises from a solvent concentration gradient that was established normal to the film surface during the solvent evaporation. Additionally, the range of conditions where the perpendicular orientation forms increased as the film thickness increased. Moreover, the PB helical microdomain, which has low affinity for the solvent, is a key factor in maintaining the orientation over a long distance. Comparison with a similar SM diblock copolymer showed that an additional block in a copolymer may result in novel structures and allow control over the orientation of the structure. This helical structure has great potential as a soft material for many new applications.
References
Bates, F. S. & Fredrickson, G. H. Block copolymers - designer soft materials. Phys. Today 52, 32–38 (1999).
Hamley, I. W. Nanostructure fabrication using block copolymers. Nanotechnology 14, R39–R54 (2003).
Kim, S. O., Solak, H. H., Stoykovich, M. P., Ferrier, N. J., de Pablo, J. J. & Nealey, P. F. Epitaxial self-assembly of block copolymers on lithographically defined nanopatterned substrates. Nature 424, 411–414 (2003).
Fasolka, M. J., Harris, D. J., Mayer, A. M., Yoon, M. & Mochrie, S. G. J. Observed substrate topography-mediated lateral patterning of diblock copolymer films. Phys. Rev. Lett. 79, 3018–3021 (1997).
Rockford, L., Mochrie, S. G. J. & Russell, T. P. Propagation of nanopatterned substrate templated ordering of block copolymers in thick films. Macromolecules 34, 1487–1492 (2001).
Karim, A., Singh, N., Sikka, M., Bates, F. S., Dozier, W. D. & Felcher, G. P. Ordering in asymmetric poly (ethylene-propylene)-poly (ethylethylene) diblock copolymer thin films. J. Chem. Phys. 100, 1620–1629 (1994).
Honeker, C. C. & Thomas, E. L. Impact of morphological orientation in determining mechanical properties in triblock copolymer systems. Chem. Mater. 8, 1702–1714 (1996).
Amundson, K., Helfand, E., Quan, X. & Smith, S. D. Alignment of lamellar block copolymer microstructure in an electric field. 1. Alignment kinetics. Macromolecules 26, 2698–2703 (1993).
Morkved, T. L., Lu, M., Urbas, A. M., Ehrichs, E. E., Jaeger, H. M., Mansky, P. & Russell, T. P. Local control of microdomain orientation in diblock copolymer thin films with electric fields. Science 273, 931–933 (1996).
Thurn-Albrecht, T., Schotter, J., Kästle, G. A., Emley, N., Shibauchi, T., Krusin-Elbaum, L., Guarini, K., Black, C. T., Tuominen, M. T. & Russell, T. P. Ultrahigh-density nanowire arrays grown in self-assembled diblock copolymer templates. Science 290, 2126–2129 (2000).
Gunkel, I., Stepanow, S., Thurn-Albrecht, T. & Trimper, S. Fluctuation effects in the theory of microphase separation of diblock copolymers in the presence of an electric field. Macromolecules 40, 2186–2191 (2007).
Ferri, D., Wolff, D., Springer, J., Francescangeli, O., Laus, M., Angeloni, A. S., Galli, G. & Chiellini, E. Phase and orientational behaviors in liquid crystalline main-chain/side-group block copolymers. J. Polym. Sci. Part B Polym. Phys. 36, 21–29 (1998).
Knoll, A., Horvat, A., Lyakhova, K. S., Krausch, G., Sevink, G. J. A., Zvelindovsky, A. V. & Magerie, R. Phase behavior in thin films of cylinder-forming block copolymers. Phys. Rev. Lett. 89, 035501 (2002).
Hamley, I. W., Castelletto, V., Lu, Z. B., Imrie, C. T., Itoh, T. & Al-Hussein, M. Interplay between smectic ordering and microphase separation in a series of side-group liquid-crystal block copolymers. Macromolecules 37, 4798–4807 (2004).
Grigorova, T., Pispas, S., Hadjichristidis, N. & Thurn-Albrecht, T. Magnetic field induced orientation in diblock copolymers with one crystallizable block. Macromolecules 38, 7430–7433 (2005).
Sakurai, S. Progress in control of microdomain orientation in blockcopolymers – efficiencies of various external fields. Polymer 49, 2781–2796 (2008).
Sivaniah, E., Hayashi, Y., Matsubara, S., Kiyono, S., Hashimoto, T., Fukunaga, K., Kramer, E. J. & Mates, T. Symmetric diblock copolymer thin films on rough substrates. Kinetics and structure formation in pure block copolymer thin films. Macromolecules 38, 1837–1849 (2005).
Turturro, A., Gattiglia, E., Vacca, P. & Viola, G.T. Free surface morphology of block copolymers: 1. Styrene-butadiene diblock copolymers. Polymer 36, 3987–3996 (1995).
Horvat, A., Lyakhova, K. S., Sevink, G. J. A., Zvelindovsky, A. V. & Magerle, R. Phase behavior in thin films of cylinder-forming ABA block copolymers: mesoscale modeling. J. Chem. Phys. 120, 1117–1126 (2004).
Mansky, P., Chaikin, P. & Thomas, E. L. Monolayer films of diblock copolymer microdomains for nanolithographic applications. J. Mater. Sci. 30, 1987–1992 (1995).
Mansky, P., Harrison, C. K., Chaikin, P. M., Register, R. A. & Yao, N. Nanolithographic templates from diblock copolymer thin films. Appl. Phys. Lett. 68, 2586–2588 (1996).
Kim, G. & Libera, M. Morphological development in solvent-cast polystyrene-polybutadiene-polystyrene (SBS) triblock copolymer thin films. Macromolecules 31, 2569–2577 (1998).
Lin, Z., Kim, D. H., Wu, X. D., Boosahda, L., Stone, D., LaRose, L. & Russell, T. P. A Rapid route to arrays of nanostructures in thin films. Adv. Mater. 14, 1373–1376 (2002).
Kim, S. H., Misner, M. J., Xu, T., Kimura, M. & Russell, T. P. Highly oriented and ordered arrays from block copolymers via solvent evaporation. Adv. Mater. 16, 226–231 (2004).
Ho, R. M., Tseng, W. H., Fan, H. W., Chiang, Y. W., Lin, C. C., Ko, B. T. & Huang, B. H. Solvent-induced microdomain orientation in polystyrene-b-poly(L-lactide) diblock copolymer thin films for nanopatterning. Polymer 46, 9362–9377 (2005).
Cavicchi, K. A. & Russell., T. P. Solvent annealed thin films of asymmetric polyisoprene−polylactide diblock copolymers. Macromolecules 40, 1181–1186 (2007).
Phillip, W. A., Hillmyer, M. A. & Cussler, E. L. Cylinder orientation mechanism in block copolymer thin films upon solvent evaporation. Macromolecules 43, 7763–7770 (2010).
Jinnai, H., Kaneko, T., Matsunaga, K., Abetz, C. & Abetz, V. A double helical structure formed from an amorphous, achiral ABC triblock terpolymer. Soft Matter 5, 2042–2046 (2009).
Jinnai, H., Spontak, R. J & Nishi, T. Transmission electron microtomography and polymer nanostructures. Macromolecules 43, 1675–1688 (2010).
Jinnai, H. & Spontak, R. J. Transmission electron microtomography in polymer research. Polymer 50, 1067–1087 (2009).
Stadler, R., Auschra, C., Beckmann, J., Krappe, U., Voigt-Martin, I. & Leibler, L. Morphology and thermodynamics of symmetric poly(A-block-B-block-C) triblock copolymers. Macromolecules 28, 3080–3097 (1995).
Brandrup, J., Immergut, E. H. & Grulke, E. A. Polymer Handbook 4th edn (John Wiley & Sons, New York, 1999).
Acknowledgements
We thank Mr Ryuhei Kimoto (Kyoto Institute of Technology) for his preliminary experiments, which initiated this work. The authors are grateful to the Ministry of Education, Science, Sports and Culture through Grants-in-Aid No. 21015017 and No. 21106512.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hong, S., Higuchi, T., Sugimori, H. et al. Highly oriented and ordered double-helical morphology in ABC triblock terpolymer films up to micrometer thickness by solvent evaporation. Polym J 44, 567–572 (2012). https://doi.org/10.1038/pj.2012.69
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pj.2012.69
Keywords
This article is cited by
-
Interfacial morphologies and associated processes of multicomponent polymers
Polymer Journal (2018)
-
Controlled incorporation behavior of gold nanoparticles into ABC triblock terpolymer with double-helical morphology
Polymer Journal (2016)
-
Interface manipulated two-phase nanostructure in a triblock terpolymer with a short middle segment
Polymer Journal (2016)