Abstract
The effects of poly(γ-glutamic acid) (γPGA), a water-soluble poly(amino acid), on the enzyme activity and stability have been investigated. The activity of carbonic anhydrase (CA) was distinctly improved in the presence of 0.30–2.0% γPGA at pH 7. γPGA was also effective to enhance the activity of lipase and α-amylase. γPGA efficiently suppressed denaturation of the enzyme by the thermal treatment and repeated freeze–thaw process. The interaction of γPGA and CA was investigated by using fluorescence probe-labeled protein. The Michaelis–Menten kinetics on the CA-catalyzed hydrolysis of p-nitrophenyl acetate in the absence or presence of γPGA were performed, which demonstrated that the enhancement of the CA activity by γPGA is ascribed to the increase of the catalytic constant kcat.
Similar content being viewed by others
Introduction
There has been an increasing interest in enzyme engineering from practical aspects owing to environmentally benign catalysis of enzymes under mild reaction conditions with regard to temperature, pressure and pH, which often lead to remarkable energy efficiency. Enzymes are useful for bioremediation of polluted water and contaminated soil, improvement of cleaning efficiency of detergents, and production of biofuel, and highly expected as potential practical applications in industry.1, 2 For these applications of enzymes, the loss of enzyme activity in use is often observed, which sometimes restricts the industrial applications. In addition, the activity is influenced by temperature and chemical environments such as pH and salt type.
For purification and isolation of enzymes, lyophilization is widely used; however, the repeated freeze–thaw process often induces denaturation of the enzyme due to multiple stresses. Once enzymes fold to their native form, they are generally stable under mild conditions. On the other hand, most of unstable enzymes readily form aggregations even under mild conditions to lose their activity. Therefore, protection of enzymes against environmental stresses is required in industrial applications.
So far, there have been various methods to stabilize enzymes3, 4, 5, 6 and enhance enzyme activity; for the latter, enzyme immobilization,4, 5, 6, 7 chemical modification of enzymes8, 9, 10 and medium engineering11, 12 were reported. Polyols,13 saccharides,13, 14 surfactants15 and water-soluble polymers, such as poly(vinyl alcohol),15 poly(ethylene glycol) (PEG),15, 16, 17 and poly(ethyleneimine),18 acted as enzyme stabilizer or enhancer of enzyme activity. Furthermore, many researchers have attempted to improve enzyme stability and to enhance enzyme activity by rational design and in vitro evolution of enzymes.19, 20, 21, 22, 23 In most cases, however, protein biocatalysts showing the desired properties have not been obtained.
Poly(γ-glutamic acid) (γPGA) is a water-soluble biopolymer consisting of D- or L-γ-glutamate unit. γPGA is produced by several Bacillus species such as Bacillus anthracis, Bacillus licheniformis and Bacillus subtilis.24, 25, 26, 27, 28 γPGA is substantially biodegradable, nontoxic to humans and even edible; thus, its industrial applications have been extensively studied from industrial standpoints. Its multifunctionalities made it a promising biopolymer for various uses such as health foods, moisturizers in cosmetics, chelating agents in wastewater treatment, hydrogels for environmental, agricultural and biomedical applications.26, 27, 28, 29, 30
This study deals with the effects of γPGA on the enzyme activity and stability. γPGA is an amino-acid polymer via peptide linkage; thus γPGA will be interacted with enzyme polypeptides to influence the enzyme action. In this study, carbonic anhydrase (CA), lipase and α-amylase were selected as target proteins, which are easy to assay their activity; they are often used as a model enzyme to examine the stability and improvement of the activity.15, 31, 32
Experimental procedure
Materials
γPGAs (sodium salt form, molecular weight: 50 and 500 kDa) were commercial products of BioLeaders Corp. (Daejeon, Korea). CA from bovine erythrocytes, Amano lipase AK from Pseudomonas fluorescens, α-amylase type VI-B from porcine pancreas, p-nitrophenyl acetate (pNPA), fluorescein isothiocyanate (FITC) isomer I and phosphate-buffered saline (pH 7.4) were purchased from Sigma-Aldrich (St Louis, MO, USA). 1-Anilinonaphthalene-8-sulfonate was a product of Tokyo Chemical Industry (Tokyo, Japan). Potassium dihydrogen phosphate (KH2PO4), potassium monohydrogen phosphate (K2HPO4), tris(hydroxymethyl)aminomethane (Tris), calcium chloride (CaCl2), p-nitrophenyl propionate (pNPP) and acetonitrile were products of Wako Pure Chemical Industries (Osaka, Japan). Sodium chloride (NaCl) was purchased from Nacalai Tesque (Kyoto, Japan). All solvents and reagents were used without further purification.
Preparation of γPGA–enzyme mixtures and assays of enzyme activity
γPGA and CA were separately dissolved in 25 mM potassium phosphate buffer (pH 7.0) and mixed, and then the resulting mixture was further diluted by this buffer to 0.030 mg ml−1 concentration of CA. The concentration of γPGA was adjusted according to each experiment. The activity of CA was determined by assay of the pNPA hydrolysis. The assay started by mixing 10 μl of 100 mM pNPA in acetonitrile and 1.0 ml of the above CA solution with different γPGA concentration for 20 s at 25 °C,33, 34, 35 and the amount of the resulting p-nitrophenol was monitored from the absorbance change at 400 nm as a function of time by U-2810 ultraviolet-visible spectrophotometer (Hitachi, Tokyo, Japan). Michaelis–Menten kinetics were performed in the concentration range of pNPA from 2.5 × 10−4 to 1.0 × 10−2 M in the fixed concentration of CA as 0.030 mg ml−1. The kinetic parameters, Km and kcat, were determined from Lineweaver–Burk plots of 1/v versus 1/[S].36
In the case of lipase, a solution of 0.030 mg ml−1 lipase including 0.50% of γPGA, 150 mM NaCl, 1.36 mM CaCl2 and 13 mM Tris–HCl was prepared and the pH of the solution was adjusted at pH 8.0. For the heat treatment, the sample was kept at 55, 70, 85 or 95 °C for 10 min and then cooled at 25 °C for 20 min. The lipase activity was determined by monitoring the amount of p-nitrophenol formed by the hydrolysis of pNPP at 25 °C.37, 38
For α-amylase, a solution of 0.060 mg ml−1 α-amylase including 0.50% of γPGA and 0.10 M of 4-morpholinepropanesulfonate was prepared and the pH of the solution was adjusted at pH 6.9. For the heat treatment, the sample was kept at 37, 50, 65 or 80 °C for 20 min and then cooled at 25 °C for 20 min. α-Amylase activity was determined according to the modified protocol of EnzChek Ultra Amylase Assay Kit (Molecular Probes, Eugene, OR, USA).
Preparation of FITC-CA
To a 5.0 mg ml−1 CA solution in 0.10 M sodium carbonate bicarbonate (pH 9.0), a 1.0 mg ml−1 FITC solution in the same buffer was added under gentle stirring and kept at 25 °C for 2 h under dark conditions. The resulting FITC-labeled CA (FITC-CA) was purified by gel filtration chromatography (2.0 × 30 cm) of Sephadex G-100 (Pharmacia Fine Chemicals, Uppsala, Sweden) with phosphate-buffered saline (pH 7.4) as eluent.39, 40 Fluorescence spectra were recorded by F-2500 fluorescence spectrophotometer (Hitachi).
Freeze–thaw process
A 1 ml volume of a 0.030 mg ml−1 CA solution without or with 1.0% γPGA-50 was transferred into 1.5 ml Eppendorf tube and frozen at −25 °C. The frozen mixture was thawed at room temperature. This process was repeated in several cycles and the enzyme activity was measured.
Results and discussion
Effect of γPGA on the activity of CA
At first, the activity of CA in the presence of 0.50% various water-soluble polymers such as γPGA with molecular weight of 50 kDa, hyaluronic acid (HA), PEG, and alginic acid (ALG) was examined in a potassium phosphate buffer of pH 7.0 (Figure 1). In this study, the concentration of CA was fixed as 0.030 mg ml−1 and pNPA was used as substrate for the activity assay of CA. Without CA, the pNPA hydrolysis hardly proceeded in the presence of γPGA, strongly suggesting that γPGA did not induce the pNPA hydrolysis (data not shown). Among the water-soluble polymers examined, only γPGA improved the activity of CA. In the case of hyaluronic acid, the activity was almost the same as that without the additive, and the presence of PEG and ALG reduced the enzymatic activity. These results suggest that γPGA acts as an enhancer of enzyme activity in a simple mixing process.
Figure 2 shows the effects of the concentration and molecular weight of γPGA on the activity of CA. γPGAs with molecular weight of 50 and 500 kDa (γPGA-50 and γPGA-500, respectively) were used. When the concentration of γPGA was very low (0.010%), the improved effect was hardly observed. In the concentration range of 0.3–2.0%, the activity increased as a function of the concentration (Table 1). The molecular weight of γPGA somewhat influenced the activity; the activity in the presence of γPGA-500 was a little larger than that in γPGA-50. In case of the γPGA concentration of 3.0%, on the other hand, the activity decrease was found in comparison with that in the γPGA concentration of 2.0%, and the reduction ratio was much larger for γPGA-500. This is probably due to the dramatic viscosity increase of the reaction mixture. The viscosity values of the mixture of γPGA-500 and CA with 2.0 and 3.0% of γPGA-500 were 7.8 and 10.5 cP, respectively. It was reported that the high viscosity of the reaction media hampered the activity of lactate dehydrogenase and ATPase.41, 42
In order to verify the effects of γPGA on the enhancement of the activity of CA, the Michaelis–Menten kinetics for the CA-catalyzed hydrolysis of pNPA were carried out in the fixed γPGA concentration of 0.50% at 25 °C. Figure 3 shows the relationships between the concentration of substrate ([S]) and the initial reaction velocity (v) in the absence and presence of γPGA. The reaction in the presence of γPGA proceeded faster than that without γPGA and the larger molecular weight γPGA was slightly more efficient to catalyze the reaction.
Table 2 summarizes the kinetic parameters determined from the Lineweaver–Burk plots (data not shown). Both values of Km and kcat obtained without γPGA-50 were smaller than those in the presence of γPGA and the effect of the molecular weight of γPGA was relatively small. The catalytic efficiency kcat/Km in the presence of γPGA was a little larger than that without γPGA and the highest efficiency was observed in the presence of γPGA-500. These results indicate that the improved activity of CA by γPGA is ascribed to the enormous increase of the reaction rate in spite of the decrease of the binding ability by the addition of γPGA.
As shown above, γPGA improved the catalytic activity of CA, which may be due to the interaction between γPGA and CA. FITC-labeled proteins are often used for the mechanistic analysis to elucidate the interaction of proteins with target molecules. Thus, FITC-CA was prepared and fluorescence spectra of a mixture of FITC-CA and various water-soluble polymers were measured (Figure 4). The molar ratio of FITC to CA determined by ultraviolet-visible spectroscopy was 1.3; thus, the surface properties of FITC-labeled might be close to CA itself.
In all cases examined, the spectrum pattern was very similar to each other and the largest fluorescence intensity was observed at 520 nm. The largest intensity in the spectrum of FITC-CA without any additives was almost the same as that with hyaluronic acid and a little smaller than that with PEG. On the other hand, the remarkable increase of the largest intensity was found by the addition of γPGA, indicating that the surrounding environment of the FITC moiety becomes hydrophobic in the presence of γPGA.43, 44 These data suggest that γPGA wraps FITC-CA by their physical interaction to locate FITC-CA in the hydrophobic region, which may lead to the improved activity of CA by γPGA. Furthermore, 1-anilinonaphthalene-8-sulfonate was used as hydrophobic probe for the investigation with γPGA. The increase of its fluorescence intensity was found by the addition of the γPGA, which supports the hydrophobic region of γPGA. The net charge and isoelectric point (pI) of CA were −1.2 and 6.7, respectively; thus CA was almost neutral at pH 7, suggesting the weak electrostatic interaction between γPGA and CA.
Effect of γPGA on the activity of lipase and α-amylase and their thermal denaturation
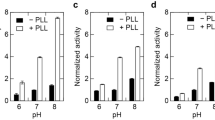
We have examined whether γPGA was effective for the activity enhancement of other enzymes (lipase and α-amylase) besides CA (Figure 5). Lipase used in this study was a heat-resistant enzyme and showed the optimum activity at 55 °C; thus, the enhanced effect was examined at 55 °C. The activity of α-amylase was measured at 25 °C. The activity of both enzyme improved by the addition of γPGA (Table 1). The enhanced ratio of α-amylase for the relative activity was 38%, which was higher than that of lipase.
The thermal denaturation effect for these enzymes by γPGA has been examined. The thermal treatment was performed at different temperatures. For lipase, the enzyme activity in the presence of γPGA-50 was higher than that in the absence γPGA-50 between 55 and 85 °C. It is to be noted that the relative activity was beyond 100% even at 70 °C by the addition of γPGA-50. More than twofold activity was found at 70 and 85 °C in comparison with that without γPGA. For α-amylase, the activity in the presence of γPGA-50 at 25 and 37 °C was much higher than that without γPGA-50. Even at 50 °C, the higher activity was found with γPGA-50. These data indicate that γPGA was effective to protect lipase and α-amylase by the thermal denaturation.
Protection effect of γPGA for freeze–thaw stress
It is reported that freeze–thaw processes seriously damage proteins irreversibly.45, 46 Here, the protection effect of γPGA for the repeated freeze–thaw process was examined by using CA as model enzyme. In the absence of γPGA-50, the activity of CA was about 65% after nine cycles and completely lost after 27 cycles (Figure 6). On the other hand, the activity was beyond 100% after nine cycles in the presence of γPGA and the activity remained even after 35 cycles. These data suggest that γPGA prevents denaturation of enzymes from stress of freeze–thaw process.
Conclusion
In this study, γPGA was found to strongly influence the enzyme activity and stability. The activity of CA, lipase and α-amylase was improved by the addition of a small amount of γPGA. Generally, the thermal treatment of enzymes decreased their activity, whereas the presence of γPGA suppressed the activity decrease in the thermal treatment at high temperature to some extent for lipase and α-amylase. Furthermore, γPGA was also efficient for the suppression of denaturation of enzymes during the freeze–thaw process. These data strongly suggest that γPGA has high potential as enhancer of enzyme activity and stabilizer for thermal treatment and freeze–thaw process.
The kinetic study in the system of CA and γPGA implied that the enhancement of the enzyme activity was due to the increase of the reaction rate and the presence of γPGA prevented the binding ability of the substrate. The formation of the specific complex between CA and γPGA, in which CA was located in the hydrophobic region, was shown by the analysis using fluorescence probe-labeled CA. These results suggest that the interaction between γPGA and enzyme proteins induces the conformational change of enzymes, leading to the activity enhancement and stability improvement for environmental stresses.
γPGA is an inexpensive biopolymer produced in industrial scale, and hence, it is highly expected to be an attractive additive in the field of enzyme industry. Our present finding that γPGA was effective for the improvement of the enzyme activity as well as that of the enzyme stability for thermal denaturation and stress of freeze–thaw process is useful for industrial enzyme engineering. Further investigations including mechanistic study on the interaction of γPGA and proteins and industrial applications of γPGA for additives of enzyme products are under way in our laboratories.
References
Kirk, O., Borchert, T. V. & Fuglsang, C. C. Industrial enzyme applications. Curr. Opin. Biotechnol. 13, 345–351 (2002).
Schmid, A., Hollmann, F., Park, J. B. & Bühler, B. The use of enzymes in the chemical industry in Europe. Curr. Opin. Biotechnol. 13, 359–366 (2002).
Ó’Fágáin, C. Enzyme stabilization-recent experimental progress. Enzyme Micro. Technol. 33, 137–149 (2003).
Mateo, C., Palomo, J. M., Fernandez-Lorente, G., Guisan, J. M. & Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzyme Micro. Technol. 40, 1451–1463 (2007).
Kim, J., Grate, J. W. & Wang, P. Nanostructures for enzyme stabilization. Chem. Eng. Sci. 61, 1017–1026 (2006).
Ispas, C., Sokolov, I. & Andreescu, S. Enzyme-functionalized mesoporous silica for bioanalytical applications. Anal. Bioanal. Chem. 393, 543–554 (2009).
Bagi, K., Simon, L. M. & Szajáni, B. Immobilization and characterization of porcine pancreas lipase. Enzyme Micro. Technol. 20, 531–535 (1997).
Yamane, T., Ichiryu, T., Nagata, M., Ueno, A. & Shimizu, S. Intramolecular esterification by lipase powder in microaqueous benzene: factors affecting activity of pure enzyme. Biotech. Bioeng. 36, 1063–1069 (1990).
Mozhaev, V. V., Melik-Nubarov, N. S., Sergeeva, M.V., Šikšnis, V. & Martinek, K. Strategy for stabilizing enzymes part one: increasing stability via their multi-point interaction with a support. Biocatalysis 3, 179–187 (1990).
Govardhan, C. P. Crosslinking of enzymes for improved stability and performance. Curr. Opin. Biotechnol. 10, 331–335 (1999).
Lee, M. Y. & Dordick, J. S. Enzyme activation for nonaqueous media. Curr. Opin. Biotechnol. 13, 376–384 (2002).
Klibanov, A. M. Improving enzymes by using them in organic solvents. Nature 409, 241–246 (2001).
Adlercreutz, P. Activation of enzymes in organic media at low water activity by polyols and saccharides. Biochim. Biophys. Acta. 1163, 144–148 (1993).
Arakawa, T. & Timasheff, S. N. Stabilization of protein structure by sugars. Biochemistry 21, 6536–6544 (1982).
Yoon, S. H. & Robyt, J. F. Activation and stabilization of 10 starch-degrading enzymes by triton X-100, polyethylene glycols, and polyvinyl alcohols. Enzyme Micro. Technol. 37, 556–562 (2005).
Simon, L. M., Kotormán, M., Garab, G. & Laczkó, I. Effects of polyhydroxy compounds on the structure and activity of α-chymotrypsin. Biochem. Biophys. Res. Commun. 293, 416–420 (2002).
Guo, W. & Mabrouk, P. A. Raman evidence that the lyoprotectant poly(ethylene glycol) does not restore nativity to the heme active site of horseradish peroxidase suspended in organic solvents. Biomacromolecules 3, 846–849 (2002).
Andersson, M. M. & Hatti-Kaul, R. Protein stabilising effect of polyethyleneimine. J. Biotechnol. 72, 21–31 (1999).
Ulmer, K. M. Protein engineering. Science 219, 666–671 (1983).
Eijsink, V. G. H., Bjørk, A., Gåseidnes, S., Sirevåg, R., Synstad, B., Burg, B. & Vriend, G. Rational engineering of enzyme stability. J. Biotechnol. 113, 105–120 (2004).
Arnold, F. H., Wintrode, P. L., Miyazaki, K. & Gershenson, A. How enzymes adapt: lessons from directed evolution. Trends Biochem. Sci. 26, 100–106 (2001).
Brannigan, J. A. & Wilkinson, A. J. Protein engineering 20 years on. Nature 3, 964–970 (2002).
Lehmann, M. & Wyss, M. Engineering proteins for thermostability: the use of sequence alignments versus rational design and directed evolution. Curr. Opin. Biotechnol. 12, 371–375 (2001).
Roelants, G. E. & Goodman, J. W. Immunochemical studies on the poly-γ-D-glutamyl capsule of Bacillus anthracis. IV. The association with peritoneal exudate cell ribonucleic acid of the polypeptide in immunogenic and nonimmunogenic forms. Biochemistry 7, 1432–1440 (1968).
Ko, Y. H. & Gross, R. A. Effects of glucose and glycerol on γ-poly(glutamic acid) formation by Bacillus licheniformis ATCC 9945a. Biotechnol. Bioeng. 57, 430–437 (1998).
Richard, A. & Margaritis, A. Poly(glutamic acid) for biomedical applications. Crit. Rev. Biotech. 21, 219–232 (2001).
Sung, M. H., Park, C., Kim, C. J., Poo, H., Soda, K. & Ashiuchi, M. Natural and edible biopolymer poly-γ-glutamic acid: synthesis, production, and applications. Chem. Record 5, 352–366 (2005).
Kim, T. W., Lee, T. Y., Bae, H.C., Hahm, J. H., Kim, Y. H., Park, C., Kang, T. H., Kim, C. J., Sung, M. H. & Poo, H. Oral administration of high molecular mass poly-γ-glutamate induces NK cell-mediated antitumor immunity. J. Immun. 179, 775–780 (2007).
Park, C., Choi, Y. H., Shin, H. J., Poo, H., Song, J. J., Kim, C. J. & Sung, M. H. Effect of high-molecular-weight poly-γ-glutamic acid from Bacillus subtilis (chungkookjang) on Ca solubility and intestinal adsorption. J. Microbiol. Biotechnol. 15, 855–858 (2005).
Lee, T. Y., Kim, Y. H., Yoon, S. W., Choi, J. C., Yang, J. M., Kim, C. J., Schiller, J. T., Sung, M. H. & Poo, H. Oral administration of poly-gamma-glutamate induces TLR4- and dendritic cell-dependent antitumor effect. Cancer Immunol. Immunother. 58, 1781–1794 (2009).
Nomura, Y., Sasaki, Y., Takagi, M., Narita, T., Aoyama, Y. & Akiyoshi, K. Thermoresponsive controlled association of protein with a dynamic nanogel of hydrophobized polysaccharide and cyclodextrin: heat shock protein-like activity of artificial molecular chaperone. Biomacromolecules 6, 447–452 (2005).
Simon, L. M., László, K., Vértesi, A., Bagi, K. & Szajáni, B. Stability of hydrolytic enzymes in water-organic solvent systems. J. Mol. Cat. B: Enzym. 4, 41–45 (1998).
Pocker, Y. & Storm, D. R. The catalytic versatility of erythrocyte carbonic anhydrase IV kinetic studies of enzyme-catalyzed hydrolyses of p-nitrophenyl esters. Biochemistry 7, 1202–1214 (1968).
Tu, C., Thomas, H. G., Wynns, G. C. & Silverman, D. N. Hydrolysis of 4-nitrophenyl acetate catalyzed by carbonic anhydrase III from bovine skeletal muscle. J. Biol. Chem. 261, 10100–10103 (1986).
Akiyoshi, K., Sasaki, Y. & Sunamoto, J. Molecular chaperone-like activity of hydrogel nanoparticles of hydrophobized pullulan: thermal stabilization with refolding of carbonic anhydrase B. Bioconjugate Chem. 10, 321–324 (1999).
Fersht, A Structure and Mechanism in Protein Science: A Guide to Enzyme Catalysis and Protein Folding, Ch. 2,103–131 (W. H. Freeman and Co.: New York, 1998).
Bastida, A., Sabuquillo, P., Armisen, P., Fernández-Lafuente, R., Huguet, J. & Guisán, J. M. A single step purification, immobilization, and hyperactivation of lipases via interfacial adsorption on strongly hydrophobic supports. Biotechnol. Bioeng. 58, 486–493 (1998).
López-Serrano, P., Cao, L., Rantwijk, F. & Sheldon, R. A. Cross-linked enzyme aggregates with enhanced activity: application to lipases. Biotechnol. Lett. 24, 1379–1383 (2002).
Dedmon, R. E., Holmes, A. W. & Deinhardt, F. Preparation of fluorescein isothiocyanate-labeled γ-globulin by dialysis, gel filtration, and ion exchange chromatography in combination. J. Bacteriol. 89, 734–739 (1965).
Shu, F., Kobayashi, H., Fukudome, K., Tsuneyoshi, N., Kimoto, M. & Terao, T. Activated protein C suppresses tissue factor expression on U937 cells in the endothelial protein C receptor-dependent manner. FEBS Lett. 477, 208–212 (2000).
Demchenko, A. P., Rusyn, O. I. & Saburova, E. A. Kinetics of the lactate dehydrogenase reaction in high-viscosity media. Biochim. Biophys. Acta. 998, 196–203 (1989).
Sampedro, J. G. & Uribe, S. Trehalose-enzyme interactions result in structure stabilization and activity inhibition. The role of viscosity. Mol. Cellular Biochem. 256, 319–327 (2004).
Aoyagi, S., Imai, R., Sakai, K. & Kudo, M. Reagentless and regenerable immunosensor for monitoring of immunoglobulin G based on non-separation immunoassay. Biosen. Bioelec. 18, 791–795 (2003).
Kim, M. S., Seo, K. S., Khang, G. & Lee, H.B. Preparation of a gradient biotinylated polyethylene surface to bind streptavidin-FITC. Bioconjugate Chem. 16, 245–249 (2005).
Park, J. I., Grant, C. M., Davies, M. J. & Dawes, I. W. The cytoplasmic Cu, Zn superoxide dismutase of Saccharomyces cerevisiae is required for resistance to freeze-thaw stress. J. Biol. Chem. 273, 22921–22928 (1998).
Pikal-Cleland, K. A., Rodríguez-Hornedo, N., Amidon, G. L. & Carpenter, J. F. Protein denaturation during freezing and thawing in phosphate buffer systems: monomeric and tetrameric β-galactosidase. Arch. Biochem. Biophys. 384, 398–406 (2000).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, EH., Tsujimoto, T., Uyama, H. et al. Enhancement of enzyme activity and stability by poly(γ-glutamic acid). Polym J 42, 818–822 (2010). https://doi.org/10.1038/pj.2010.71
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pj.2010.71
Keywords
This article is cited by
-
Activation of L-lactate oxidase by the formation of enzyme assemblies through liquid–liquid phase separation
Scientific Reports (2023)
-
Improvement Thermal Stability of d-Lactate Dehydrogenase by Hydrophobin-1 and in Silico Prediction of Protein–Protein Interactions
Molecular Biotechnology (2021)
-
Development of a Safe Solid-State Microorganism/Biodegradable Polymer Composite for Decomposition of Formaldehyde
Journal of Polymers and the Environment (2014)