Abstract
The purpose of this study was to investigate the effect of additives on the recycling of waste polycarbonate (PC) by hydrolysis in a steam atmosphere. PC containing a variety of additives was obtained from waste electrical and electronic equipment (WEEE) and was hydrolyzed at 300 and 500 °C in a semibatch reactor in the presence of MgO and CaO. Valuable phenolic products were obtained at high yield, with a product distribution that was strongly dependent on temperature. Bisphenol A (BPA) was the major product formed from the hydrolysis of waste PC at 300 °C and was obtained in a maximum yield of 91%, and degradation products of BPA such as phenol and 4-isopropenyl phenol were obtained at 500 °C. The presence of polystyrene and triphenyl phosphate in waste PC reduced the rate of the reaction by preventing steam from interacting with the surface of PC. Although pure PC was completely hydrolyzed within 15 min, hydrolysis of waste PC took 30–60 min. However, the hydrolysis of PC in a steam atmosphere is an appropriate method for materials that cannot be treated by solvolysis.
Similar content being viewed by others
Introduction
Polycarbonate (PC) is a plastic used in the construction of various items such as computer housings, headlights, household devices, CDs and other applications that require excellent optical properties. The PC from such items can be collected in relatively large pieces and separated from other waste to be recycled into relatively homogeneous material. PC can be recycled using solvolysis1, 2 or mechanical recycling, but neither of these methods is suitable if high concentrations of additives are present because of contamination of the recyclate. Pyrolysis of PC results in the production of high volumes of low-value char and gases. Therefore, alternative methods of recycling PC are desired.
The backbone of PC, bisphenol A (BPA), is degraded by high temperature3 or supercritical water,4 leading to the formation of phenols. Therefore, hydrolysis, which combines both high temperature and the presence of water-based radicals, is a promising method for recycling PC that contains additives or is otherwise unsuitable for mechanical recycling. Earlier results5, 6 have shown that the hydrolysis of PC in a steam atmosphere is strongly accelerated by the presence of alkali earth oxides and hydroxides, resulting in high yields of BPA at 300 °C. At 500 °C, phenol and 4-isopropenyl phenol (IPP) are formed by the decomposition of BPA. Studies have shown that MgO is more active than CaO in the hydrolysis of PC, whereas differences between oxides and hydroxides were negligible.
Hydrolysis may be particularly useful for the recycling of PC from waste electrical and electronic equipment (WEEE). WEEE plastics contain various additives that reduce the flammability of the polymer. These additives may include brominated and/or phosphorylated flame retardants and synergists such as antimony trioxide. A classic flame retardant added to PC is tetrabromobisphenol A, but triphenyl phosphate (TPP) is often used because it functions as a plasticizer and flame retardant. In addition to flame retardant additives, WEEE plastics also contain significant quantities of metals derived from fillers such as calcium carbonate and the material recovery process, in which an imperfect separation of shredded plastic and metal components leads to metal contamination in the plastic recyclate.
In this study, we investigated the use of hydrolysis for the recycling of PC from a WEEE-processing plant in the presence of magnesium and calcium catalysts. Hydrolysis products were characterized by gas chromatography–mass spectrometry and the yield of valuable products was determined.
Experimental procedure
Material
Waste PC was obtained as a mixture from a WEEE-processing plant in Peterborough (UK) and was derived from vacuum cleaners, telephones and other electrical devices. TiO2 (1.8 wt%), phosphorylated flame retardants/plasticizers (6.6 wt% as TPP) and brominated flame retardants (0.15 wt% as tetrabromobisphenol A) were identified in waste PC. In addition, aluminum (0.1 wt%) was present as a contaminant in the recycling process. The PC content of the mixture was 87 wt% and polystyrene (5 wt%) was present as a polymer blend.
Ethanol, naphthalene, CaO and MgO (all special grade) were obtained from Kanto Kagaku (Tokyo, Japan).
Hydrolysis experiment
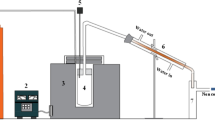
Experiments were conducted in a semibatch fixed bed reactor (Figure 1) at temperatures between 300 and 500 °C under an 86 mol% steam atmosphere in a helium carrier gas. Steam was generated at 150 °C and piped through an isolated tube into the reactor. Product oil was collected in traps cooled with iced water and liquid nitrogen, and product gas was collected in a gas bag. All experiments were repeated to verify the results.
Samples consisting of 230 mg of PC and 180 mg of MgO or 250 mg of CaO (molar ratio of 1:5) were fed into a hot reactor zone over a period of 10 min to ensure a high sample heating rate. After reactants were fed into the reactor, the temperature was maintained at 500 °C for 20 min or at 300 °C for 50 min. The reaction was assumed to be complete within the indicated time frame because longer reaction times did not lead to significantly different results. When the reaction was complete, the reactor was purged for 5 min with helium and the reactor and cooling traps were washed with ethanol. The collected solution was filtered through a G5 frit to remove any solid residue.
Analysis
Gas composition was analyzed by GC-TCD (GC-323, GL Science, Tokyo, Japan) with a packed column (Carboplot P7, Varian, Palo Alto, CA, USA: 50 °C (3 min) → 10 °C min−1 → 150 °C (3 min)) and the condensed products were analyzed qualitatively by gas chromatography–mass spectrometry (GC: Hewlett Packard HP6890, MS: Hewlett Packard HP5973, Palo Alto, CA, USA) with a CP-Sil 24 MS capillary column. Quantitative analysis was performed on a GC-FID (GL-Science GC-390) with a capillary column (CP-Sil 24 CB). Response factors of the products were calculated according to the method of Jorgensen et al.7 Naphthalene was used as an internal standard. An identical temperature program (50 °C (5 min) → 10 °C min−1 → 320 °C (5 min)) was used in GC-FID and gas chromatography–mass spectrometry analysis. The difference between the mass of products and the mass of organic material in WEEE-PC samples was defined as the mass balance (98.1 wt%).
WEEE-PC samples were examined by XRD and XRF to identify additives. XRD was also used to analyze residual inorganic material (Rigaku Denki Geiger-flex 2013, Rigaku Denki, Tokyo, Japan).
Results and discussion
Hydrolysis of the PC fraction of WEEE was similar to the hydrolysis of pure PC.6 However, the reaction time was significantly longer. Although the hydrolysis of pure PC was completed within 15 min at either temperature, hydrolysis of PC derived from WEEE took 60 and 30 min at 300 and 500 °C, respectively. This behavior might have been caused by foreign substances in WEEE-PC samples, such as polystyrene and phosphorylated flame retardants. Although polystyrene and phosphorylated additives display different behavior during hydrolysis, the presence of either chemical may yield similar results. Flame-retardant properties of phosphorous acid derivatives are a result of radical quenching in the gas phase and because of the formation of a nonvolatile thermoprotective solid layer that prohibits the formation of gases.8, 9 At 500 °C, reactions between phenols and phosphates might have resulted in the formation of branched PC, reducing the rate of hydrolysis. Polystyrene, on the other hand, was relatively unaffected by steam at temperatures below 400 °C (Figure 2). Given that PC particles shrank during hydrolysis, it can be assumed that polystyrene was enriched on the surface of PC, hindering contact with the atmosphere and reducing the rate of hydrolysis.
Reaction products
The products of WEEE-PC hydrolysis were identical to those of pure PC. Although phenol and IPP were observed as fission products of BPA at 300 °C, BPA was the major product of hydrolysis (Figure 2). The production of phenol and IPP increased at higher temperatures, whereas the amount of BPA produced by hydrolysis varied slightly between 300 and 450 °C. At 500 °C, fission products of BPA became dominant and phenol and IPP were obtained as the major products. Styrene was also obtained at 500 °C through pyrolysis of the polystyrene present in the sample.
Pyrolytic reactions were evidenced by changes in the appearance of product oil and residue (Figure 3). The oil changed from colorless at 300 °C to yellow at 500 °C, and the color of the residue changed from slightly pink to black over the same temperature range. The color changes indicated an increase in pyrolytic degradation with increasing temperature. The slight pink color of the residue may be the result of trace amounts of iron (0.02 wt%) present in the PC. No significant differences were observed between liquid samples hydrolyzed in the presence of MgO and CaO.
The mass balance of the hydrolysis of WEEE-PC at 300 and 500 °C is presented in Table 1. At 300 °C, BPA was the major product and was obtained at 73% yield by weight. This is very similar to the hydrolysis of pure PC, which yielded 70 wt% BPA. However, less 4-α-cumyl phenol and more phenol and IPP were formed in WEEE-PC compared with pure PC (phenol: not detected, IPP: 1.2 wt%, 4-α-cumyl phenol: 2.7 wt%).6 A negative mass balance at 300 °C using MgO as a catalyst was attributed to the complete hydrolysis of PC and TPP. The organic content of WEEE-PC was defined as 100 wt%; therefore, the complete hydrolysis of WEEE content would have resulted in a product yield of ∼107 wt%. After considering the theoretical yield, ∼5 wt% of material remained unaccounted for and was attributed to the concentration of styrene in WEEE-PC. Yields obtained in the presence of CaO were significantly lower at 300 °C. The hydrolysis results of WEEE-PC and pure PC were also similar at 500 °C. Higher weight ratios of phenol and IPP and lower ratios of BPA and 4-α-cumyl phenol were produced from WEEE-PC in the presence of MgO. The hydrolysis of pure PC in the presence of Mg(OH)2 yielded 14, 21, 26 and 2.6 wt% of BPA, phenol, IPP and 4-α-cumyl phenol, respectively.6 These results revealed that the fission of BPA and degradation of IPP increased in WEEE-PC compared with pure PC.
Product yields
The yield of each product was calculated as the ratio of obtained mass (actual yield) to theoretical yield (Table 2):

The theoretical yield of BPA was defined as the mass of BPA achieved from the complete hydrolysis of PC without any further reaction (Scheme 1). The theoretical yield of IPP was defined as the mass of IPP obtained after the complete fission of BPA without any further degradation.
To calculate the yield of phenol, it was assumed that phenol was produced by the fission of BPA and hydrolysis of TPP. The degradation of IPP also results in the formation of phenol but was not considered in the calculation. It was assumed that WEEE-PC contained 87 PC and 6.6 wt% TPP; however, the amount of TPP was only an estimate based on the phosphorous content, obtained by elemental analysis. Because other compounds containing phosphorus may have been present in WEEE-PC, the actual yield of phenol might be slightly higher.
BPA was recovered in 91% yield from the hydrolysis of WEEE-PC at 300 °C in the presence of MgO. The yields of phenol and IPP were 24 and 12%, respectively. A theoretical yield of approximately 15% is achieved on complete hydrolysis of TPP. Under the assumption that IPP does not undergo further degradation to produce phenol, the theoretical yield of IPP and phenol should be 12%. Therefore, the additional phenol produced by the reaction was assigned to the hydrolysis of TPP. The total yield of BPA and IPP was 103%, which may be attributed to analytical inaccuracies. On the basis of these results, it can be assumed that the reaction in the presence of MgO led to the complete hydrolysis of PC. On the other hand, less product was obtained by the hydrolysis of WEEE-PC in the presence of CaO. Using the aforementioned analysis, 2% phenol was obtained from the fission of BPA and 9% from the hydrolysis of TPP. Adding the BPA yield (59%) and the IPP yield (2%) shows that 39% of PC was not hydrolyzed under the reaction conditions, nor did it undergo competitive reactions.
In the presence of MgO at 500 °C, BPA was degraded mainly to phenol and IPP, in yields of 73 and 42%, respectively. The total theoretical yield of IPP and phenol is >200%. Under the assumption that TPP was completely hydrolyzed under the reaction conditions, the difference between the amounts of phenol obtained from PC and IPP was 18%, suggesting that 9% of IPP was degraded. Thus, 53% of PC was degraded to phenol and IPP, 10% led to the production of BPA and 37% underwent competitive reactions and was obtained as residue. The differences between MgO- and CaO-catalyzed reactions were less significant at 500 °C than at 300 °C.
WEEE-PC required longer reaction times because the rate of hydrolysis was reduced compared with pure PC. Accordingly, more fission of BPA and IPP occurred during the hydrolysis of WEEE-PC, resulting in an increased yield of phenol.
Residue analysis
A critical factor in feedstock recycling is the presence of residue remaining after the reaction. Residue, which is considered waste, is obtained at the expense of the desired product. It requires expensive removal from the reactor and reduces the activity of the catalyst. The amount of residue produced by hydrolysis of WEEE-PC was not accessible; therefore, mass lost during the reaction was considered residue. At 300 °C, in the presence of MgO, the mass balance was negative, indicating that complete hydrolysis of PC had occurred (Table 1). As the mass balance was calculated on the basis of WEEE-PC, a negative amount of residual material indicated that the weight of the products was higher than that of the input material. The increase in mass may be due to side reactions with water during the hydrolysis of PC. In addition, polystyrene has a high degradation temperature and remained unaffected in the residue. Higher temperatures led to an increase in residual material because condensation reactions competed with the hydrolysis of PC. During condensation, aromatic structures such as diphenylether, xanthone, dibenzofuran and fluorenone were formed from neighboring PC units.10, 11 These structures are not valuable products and function as precursors for char formation. The production of condensed aromatic compounds is supported by the change in residue color from slightly pink at 300 °C to black at 500 °C (Figure 3b), indicating the presence of carbon.
One important reason for the enhanced formation of residue in the presence of CaO is the absorption of CO2. MgO does not form Mg(OH)2 or MgCO3 above 300 °C, and no changes in the catalyst were observed using XRD (Figure 4). On the contrary, CaO forms mainly Ca(OH)2 under a steam atmosphere at 500 °C. However, small amounts of CaO and CaCO3 were found at 500 °C. TiO2 was also present in all samples. Details of the degradation mechanism were presented in a previous report.6
Conclusion
Hydrolysis of the PC fraction of WEEE is not significantly different from hydrolysis of pure PC. The presence of MgO changes the mechanism of the reaction and is a more active catalyst than CaO. Styrene and TPP in impure PC causes a reduction in the reaction rate; however, styrene does not degrade at 300 °C and remains in the residue. On the other hand, styrene undergoes pyrolysis at 500 °C. TPP forms a protective layer at 500 °C, and both styrene and TPP may cover the surface of PC particles during hydrolysis and prevent steam from interacting with PC.
PC is completely hydrolyzed at 300 °C, resulting in a high yield of BPA (91%), phenol and IPP byproducts. TPP was a major source of phenol at 300 °C. Alternatively, BPA decomposes at 500 °C and phenol and IPP were obtained as the major products. Pyrolysis at 500 °C leads to an enhanced formation of residue.
Inorganic materials such as fillers, fire retardants and other additives are often present in waste PC; dirt is another common impurity. As these materials are virtually insoluble in solvents, sludge is formed during solvolysis. The separation of sludge from solvent is a complicated problem for many waste materials. In hydrolysis, inorganic solid impurities remain with the catalyst and can be separated from organic products. After hydrolysis, the catalyst can be used in construction materials if toxic substances are not present. However, if valuable materials have accumulated in the residue, it may be necessary to reprocess the catalyst. The results of this study revealed that hydrolysis can transform PC contaminated with foreign materials such as fillers, flame retardants, copolymers and blends into valuable products. Highly filled, contaminated or blended materials cannot be processed by solvolysis because extraneous substances might cause competitive reactions or prevent the separation of products. In these cases, hydrolysis is an adequate alternative to solvolysis.
References
Pinero, R., Garcia, J. & Cocero, M. J. Chemical recycling of polycarbonate in a semi-continuous lab-plant. A green route with methanol and methanol-water mixtures. Green Chem. 7, 380–387 (2005).
Hu, L.- C., Oku, A. & Yamada, E. Alkali-catalyzed methanolysis of polycarbonate. A study on recycling of bisphenol A and dimethyl carbonate. Polym. 39, 3841–3845 (1998).
Hunter, S. E. & Savage, P. E. Kinetics and mechanism of p-isopropenylphenol synthesis via hydrothermal cleavage of bisphenol A. J. Org. Chem. 69, 4724–4731 (2004).
Adschiri, T., Shibata, R. & Arai, K. Phenol recovery by bisphenol-A (BPA) tar hydrolysis in supercritical water. Sekiyu Gakkaishi 40, 291–297 (1997).
Yoshioka, T., Sugawara, K., Mizoguchi, T. & Okuwaki, A. Chemical recycling of polycarbonate to raw materials by thermal decomposition with calcium hydroxide/steam. Chem. Lett. 34, 282–283 (2005).
Grause, G., Sugawara, K., Mizoguchi, T. & Yoshioka, T. Pyrolytic hydrolysis of polycarbonate in the presence of earth-alkali oxides and hydroxides. Polym. Degrad. Stab. 94, 1119–1124 (2009).
Jorgensen, A. D., Picel, K. C. & Stamoudis, V. C. Prediction of gas chromatography flame ionization detector response factors from molecular structures. Anal. Chem. 62, 683–689 (1990).
Jang, B. N. & Wilkie, C. A. The effects of triphenylphosphate and recorcinolbis(diphenylphosphate) on the thermal degradation of polycarbonate in air. Thermochim. Acta 433, 1–12 (2005).
Bozi, J., Czégény, Z., Mészáros, E. & Blazsó, M. Thermal decomposition of flame retarded polycarbonates. J. Anal. Appl. Pyrolysis 79, 337–345 (2007).
Puglisi, C., Samperi, F., Carroccio, S. & Montaudo, G. MALDI-TOF investigation of polymer degradation. Pyrolysis of poly(bisphenol A carbonate). Macromolecules 32, 8821–8828 (1999).
Puglisi, C., Sturiale, L. & Montaudo, G. Thermal decomposition processes in aromatic polycarbonates investigated by mass spectrometry. Macromolecules 32, 2194–2203 (1999).
Acknowledgements
This research was partially supported by the Ministry of Education, Science, Sports, and Culture through Grant-in-Aid for Scientific Research (A), 21241018, 2009.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Grause, G., Tsukada, N., Hall, W. et al. High-value products from the catalytic hydrolysis of polycarbonate waste. Polym J 42, 438–442 (2010). https://doi.org/10.1038/pj.2010.21
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pj.2010.21
Keywords
This article is cited by
-
Characteristics of the steam degradation of poly(lactic acid) and poly(3-hydroxybutyrate-co-3-hydroxyhexanoate)
Polymer Journal (2024)
-
Concentrated Sulfuric Acid as a Catalyst for Chemical Recycling of Polycarbonate in Water
Waste and Biomass Valorization (2024)
-
Theoretical study of transesterification of diethyl carbonate with methanol catalyzed by base and Lewis acid
Theoretical Chemistry Accounts (2019)
-
Selective phenol recovery via simultaneous hydrogenation/dealkylation of isopropyl- and isopropenyl-phenols employing an H2 generator combined with tandem micro-reactor GC/MS
Scientific Reports (2018)
-
Feedstock recycling of waste polymeric material
Journal of Material Cycles and Waste Management (2011)