Abstract
Optically pure, helical poly(quinoxaline-2,3-diyl)s (degree of polymerization, n=10–100), prepared by asymmetric living polymerization of 1,2-diisocyano-3,6-dimethyl-4,5-di(propoxymethyl)benzene with an optically pure organopalladium initiator, formed stable monolayers on a water surface. As the polymerization degree increased, more closely packed monolayers were formed, as indicated by surface pressure-area (π-A) isotherms. Up to 54 monolayers were deposited onto the solid support using the Langmuir–Blodgett (LB) technique. Polarized ultraviolet spectra of LB films showed that the helical axis was orientated parallel to the dipping direction. This optical property indicates that polymer molecules are organized anisotropically in LB films.
Similar content being viewed by others
Introduction
Well-ordered assemblies of molecules are highly important for the ultimate design of functional materials. Even if a molecule possessing potentially intriguing properties is synthesized on the basis of appropriate design, the bulk materials prepared from the molecule often fail to exhibit desired functions because of a lack of appropriate assembling. The Langmuir–Blodgett (LB) technique is one of the most efficient methods for assembling organic molecules two dimensionally within a monolayer.1, 2, 3 By virtue of the monolayer-to-monolayer deposition processes, film materials with desired thickness are easily prepared, keeping the two-dimensional regularity of the molecular orientation.
Unlike traditional LB films that are formed from low-molecular-weight compounds, it is known that long hydrophobic alkyl chains are not necessary for the formation of stable polymer LB films.4, 5 Instead, attractive interactions between polymer backbones through a two-dimensional hydrogen bonding network, as well as through van der Waals forces, are proposed to be highly important for proper LB assembly, in which polymer molecules are tightly packed in a well-ordered manner. This suggests that perfectly linear-shaped polymer backbones, for example, rigid-rod-like polymers and extended forms of flexible polymers, are highly suitable for the formation of stable LB films, as such linear-shaped polymers can be packed effectively in the two-dimensional water surface.6, 7, 8, 9, 10, 11 In fact, one of us found that poly(N-alkylacrylamide)s adopted a perfectly extended, zigzag conformation in stable LB films, in spite of the fact that the polymer was conformationally flexible in solution.4, 12
We have developed a new chemistry of poly(quinoxaline-2,3-diyl)s that are prepared by the living polymerization of 1,2-diisocyanobenzenes.13, 14, 15, 16, 17, 18, 19, 20 Poly(quinoxaline-2,3-diyl)s are a unique class of helical polymers prepared by the living polymerization of o-diisocyanobenzenes.13 They feature an exceptionally robust helical structure that shows no change even at 80 °C in solution for several days.21 The structural analyses of the polymers indicated that they adopted highly homogeneous helical structures without any defects, such as random conformation or helix inversion at any part of the polymer chain. This proposed helical structure was supported by our experiments, in which the stereoselective synthesis of optically pure poly(quinoxaline-2,3-diyl)s was achieved by polymerization with chiral binaphthylpalladium initiators.21, 22, 23 The most striking feature of these polymers is the high screw-sense excess, which relies on the chiral terminal group derived from the chiral initiator for asymmetric living polymerization.22 With these unique characteristics, we have studied the application of a polyquinoxaline scaffold to new chiral polymer catalysts.24
The homogeneity of the helical structure of poly(quinoxaline-2,3-diyl)s was strongly indicated by the fact that the optically active binaphthyl group, which stays at the polymer end opposite to the living palladium termini during polymerization, can successfully control the helical sense of the long polymer chain. This perfect helical structure also suggests that whole polymer molecules may be regarded as perfect rigid-rod shapes without any turns. This structural feature prompted us to start an investigation into the LB assembly of poly(quinoxaline-2,3-diyl)s. Herein, we describe the first successful formation and the properties of the hierarchical LB film of helical poly(quinoxaline-2,3-diyl)s.
Experimental procedure
A series of optically pure poly(quinoxaline-2,3-diyl)s, designated as 1a–e, were prepared by the reaction of (S)-bis(dimethylphenylphosphine)iodo[3-(7′-methoxy[1,1′-binaphthalene]-2-yl)-5,8-bis(4-methylphenyl)-2-quinoxalinyl]palladium with 10 (for 1a), 20 (for 1b), 30 (for 1c), 40 (for 1d) and 100 (for 1e) equivalents of 1,2-diisocyano-3,6-dimethyl-4,5-di(propoxymethyl)benzene, according to the reported procedure (Scheme 1).21 The use of the 7′-methoxy-1,1′-binaphthyl group as initiator allows us to perform a highly screw-sensitive and molecular-weight-controlled polymerization of 1,2-diisocyanobenzenes at room temperature;21 1d and 1e, for example, have number-averaged molecular weights (Mn) of 8.8 × 103 (Mw/Mn=1.21) and 2.4 × 104 (Mw/Mn=1.15), respectively.
Measurements of surface pressure-area (π-A) isotherms and monolayer deposition were taken using an automatically controlled Langmuir trough (HBM-AP, Kyowa Interface Science Co. Ltd, Tokyo, Japan) equipped with a Wilhelmy balance. Optically pure poly(quinoxaline)s 1a–e were spread from their chloroform solutions (1 mmol dm−3) on a pure water (Milli-Q II, Nihon Millipore K.K., Tokyo, Japan) surface for the measurement of π-A isotherms. LB multilayers were deposited on quartz slides that were cleaned in boiling HNO3, washed with pure water and made hydrophobic with octadecyltrichlorosilane. LB films were deposited on solid substrates at a surface pressure and temperature of 14 mN m−1 and 20°C, respectively.
X-ray diffraction spectra were obtained on an X-ray diffractometer (M18XHF22-SRA, MAC Science Co. Ltd, Tokyo, Japan) equipped with a copper anode operating at 40 kV and 200 mA as the X-ray source (wavelength λ=1.542 Å). Ultraviolet (UV)-visible spectra were measured with a Hitachi U-3000 UV-visible spectrophotometer (Hitachi Ltd., Tokyo, Japan). CD spectra were measured with a Circular Dichroism J-720 spectrophotometer (JASCO Co. Ltd, Tokyo, Japan).
Results and discussion
Monolayer behavior on water surface
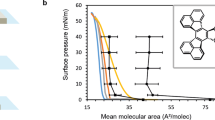
Optically pure poly(5,8-dimethyl-6,7-di(propoxymethyl)-quinoxaline-2,3-diyl)s (P)-1d (n=40, Scheme 1) were spread from a chloroform solution onto a water surface to measure π-A isotherm at 20 °C (Figure 1, Table 1). The isotherm indicates that (P)-1d forms a stable monolayer with a steep rise in surface pressure and a high collapse pressure, regardless of the absence of long alkyl chains. The shape of the π-A isotherm of (P)-1d did not change with varying temperatures (15, 20 and 24 °C) (Figure 1a). Poly(quinoxaline-2,3-diyl)s (P)-1 with different polymerization degrees were also subjected to monolayer formation at 20 °C, exhibiting a significantly different π-A isotherm (Figure 1b). Polymer (P)-1c with almost the same polymerization degree showed an essentially identical π-A isotherm to that of (P)-1d. It is noteworthy that poly(quinoxaline)s ((P)-1e) of higher polymerization degrees (n=100) showed a smaller surface area with slightly sharper increases in surface pressure. On the other hand, those with smaller polymerization degrees ((P)-1a (n=10) and (P)-1b (n=20)) showed larger surface areas with relatively gradual increases in surface pressure. These findings indicate that a better packing of molecules in the monolayer was achieved for higher molecular-weight poly(quinoxaline)s than for those with low polymerization degrees.
By analogy, with the two-dimensional molecular orientation proposed for the monolayer of poly(N-alkylacrylamide)s, we can presume a similar side-on orientation of rod-like shaped poly(quinoxaline-2,3-diyl)s (Figure 2). On the basis of the π-A isotherm of (P)-1e (n=100), which forms the most closely packed monolayer, we were able to estimate the molecular packing of poly(quinoxaline-2,3-diyl)s. From the X-ray crystal structure of oligoquinoxaline that we reported, the axis length per monomer unit is assumed to be 0.215 nm. However, the π-A isotherm of (P)-1e shows a surface area of 0.248 nm2 per monomer unit (Table 1). From these values, the diameter of the helical rod in the monolayer is calculated to be 1.15 nm. The radius (0.58 nm) approximately corresponds to the distance, by the CPK model, between the center of the helical rod and the benzylic carbon atom of the propoxymethyl side chain. This result indicates that the poly(quinoxaline) molecules in the monolayer are packed closely side by side, which could entangle the propoxymethyl side chains on the quinoxalines.
The X-ray diffraction measurements of monolayers showed a broad but significant Bragg peak of ∼2θ=5.656°. This corresponds to a layer periodicity of 1.56 nm, which was also nearly consistent with the diameter of the helical rod polymer calculated by the CPK model.
LB film formation
The monolayers of (P)-1d were successfully transferred onto a solid support (quartz slide) by both downward and upward strokes at a deposition pressure of 14 mN m−1. At this deposition pressure, the transfer ratios of the upward and downward strokes were 1.08 and 1.16, respectively. It was difficult to maintain the monolayer of (P)-1d constant at pressures over 16 mN m−1, even if the monolayer could be deposited onto the solid support. Moreover, the (P)-1d monolayer could not be deposited at pressure less than 10 mN m−1.
The UV absorption spectra of (P)-1d LB films with various numbers of deposited layers were measured (Figure 3). It is important to note that absorbance increased in an almost linear manner as the number of layers increased (Figure 3). The observed deviation from linearity may be due to a gradual decrease in the transfer ratio of the downward stroke as the number of deposited layers increased, in contrast to the constant transfer ratio for the upward stroke. The spectra also showed a slight red shift in the absorption of (P)-1d in LB films in comparison with that of (P)-1d in solution. This may indicate some interaction between chromophores due to the close polymer packing in LB films, such as J-aggregates, which are often observed in LB films.
It should be noted that the (P)-1d LB film exhibited a CD spectrum significantly different from that observed in solution (Figure 4). The CD spectrum of (P)-1d in solution (Figure 4a) showed negative and positive Cotton effects at 270 and 320 nm, respectively. Figure 4b shows CD spectra of the (P)-1d LB film measured at a certain angle without any data correction. In the CD measurements of the (P)-1d LB film, however, no Cotton effect was observed above 250 nm. As Kuball pointed out, CD spectra of anisotropic films25, 26, 27 include unavoidable effects of both linear dichroism and linear birefringence.28 Symmetrical CD spectra were observed for an LB film of (S)-1d, the enantiomer of (P)-1d; the CD spectra show the same rotations as those in solution with the opposite sign, which can be considered identical to those in solution (Figure 4a). Although a more detailed analysis is required to prove significant changes in CD spectra, we believe that poly(quinoxaline-2,3-diyl) LB films retain helical structures in the LB film and that any significant changes in the interaction of chromophores result from well-organized arrangements in LB films.
Anisotropic optical properties of LB films
Polarized UV spectra of the LB films of (P)-1d were measured for varying angles between the plane of polarization and the dipping direction for the LB film preparation. As shown in Figure 5, UV spectra changed with each angle, exhibiting less-intensive absorptions at 295 nm (26% decrease) and 355 nm (36% decrease) for angle 0° than for 90°, whereas almost the same absorbance was observed at 214 nm for both spectra at 0° and 90°. The observed angle dependence of the absorbance at 295 nm for LB films with 8, 28 and 54 layers suggested the anisotropic arrangement of polymer molecules in LB films (Figure 6a). In particular, it may be noted that LB films composed of 28 and 54 layers showed less-intense absorbance at 180° than that at 0°, whereas the eight-layer film showed the same absorbance at 0 and 180°. These differences in absorbance for 28- and 54-layer films may be due to a less-ordered deposition of polymer molecules onto films during downward stroke than during upward stroke.
We also observed a dependence of λmax near 295 nm on the sample orientation in the polarized UV measurement (Figure 6b). λmax was shifted to the longest wavelength (ca. 299 nm) at 90° and 270° and to the shortest wavelength (ca. 289 nm) around 0° and 180°. As mentioned above, the red shift implies the J-aggregate-like formation in the LB film. These observations of anisotropic optical properties indicate a well-ordered assembly of poly(quinoxaline-2,3-diyl)s in LB films.
Conclusions
We found that poly(quinoxaline-2,3-diyl)s form stable monolayers on a water surface. The measurement on π-A isotherms revealed that the polymerization degree had a critical influence on the surface area and that polymer molecules were well organized on a water surface with side-on orientation and side-by-side packing. The monolayer was successfully transferred onto solid supports as Y-type LB films. LB films have a well-defined layer structure that is 1.5 nm thick. Measurements of polarized UV spectra showed anisotropic properties of LB films, indicating an ordered orientation of polymer molecules in LB films. The optically active LB films will be good candidates for chiral discrimination and chiral sensor device application.
References
Blodgett, K. B. & Langmuir, I. Built-up films of barium stearate and their optical properties. Phys. Rev. 51, 0964 (1937).
Gaines, G. L. Insoluble Monolayers at Liquid-Gas Interfaces (Interscience Publishers, New York, 1966).
Ulman, A. An Introduction to Ultrathin Organic Films: From Langmuir-Blodgett to Self-Assembly (Academic Press, San Diego, 1991).
Taniguchi, T., Yokoyama, Y. & Miyashita, T. Spreading behavior of short-branched N-alkylacrylamide polymers and the formation of Langmuir-Blodgett films. Macromolecules 30, 3646 (1997).
Feng, F., Aoki, A. & Miyashita, T. A new type of cyclic alkylacrylamide polymer Langmuir-Blodgett film. Chem. Lett. 27, 205 (1998).
Hagting, J. G., de Vos, R., Sinkovics, K., Vorenkamp, E. J. & Schouten, A. J. Langmuir-Blodgett mono- and multilayers of (Di)alkoxy-substituted poly(p-phenylenevinylene) precursor polymers. 1. Langmuir monolayers of homo- and copolymers of (di)alkoxy-substituted precursor PPVs. Macromolecules 32, 3930 (1999).
Wegner, G. Nanocomposites of hairy-rod macromolecules: concepts, constructs, and materials. Macromol. Chem. Phys. 204, 347 (2003).
Isemura, T., Hotta, H. & Miwa, T. Surface chemistry of high polymers. I. Non-electrolytic flexible linear polymers at air/water interface. Bull. Chem. Soc. Jpn. 26, 380 (1953).
Teerenstra, M. N., Klap, R. D., Bijl, M. J., Schouten, A. J., Nolte, R. J. M., Verbiest, T. & Persoons, A. Preparation of Langmuir-Blodgett mono- and multilayers of copolymers of isocyanides with NLO-active side chains. Effect of a spacer group between the NLO chromophore and the polymer backbone. Macromolecules 29, 4871 (1996).
Kumaki, J., Kawauchi, T. & Yashima, E. Two-dimensional folded chain crystals of a synthetic polymer in a Langmuir-Blodgett film. J. Am. Chem. Soc. 127, 5788 (2005).
Ren, Y. Z., Tian, Y. Q., Sun, R. P., Xi, S. Q., Zhao, Y. Y. & Huang, X. M. Structure and photoisomerization of the Z-type Langmuir-Blodgett films of a new series of azo-containing chiral copolymers. Langmuir 13, 5120 (1997).
Miyashita, T. Recent studies of functional ultrathin polymer-films prepared by the Langmuir-Blodgett technique. Prog. Polym. Sci. 18, 263 (1993).
Ito, Y., Ihara, E., Murakami, M. & Shiro, M. New living polymerization of 1,2-diisocyanoarenes via (quinoxalinyl)palladium complexes—synthesis of poly(2,3-quinoxaline). J. Am. Chem. Soc. 112, 6446 (1990).
Ito, Y., Ihara, E., Uesaka, T. & Murakami, M. Synthesis of novel thermotropic liquid-crystalline poly(2,3-quinoxaline)s. Macromolecules 25, 6711 (1992).
Ito, Y., Ihara, E. & Murakami, M. Living polymerization of 1,2-diisocyanoarenes promoted by (quinoxalinyl)nickel complexes. Polym. J. 24, 297 (1992).
Ito, Y., Ihara, E. & Murakami, M. Enantioselective polymerization of 1,2-diisocyanoarenes—synthesis of optically-active, helical pol(quinoxaline-2,3-diyl)s. Angew. Chem. Int. Ed. 31, 1509 (1992).
Ito, Y., Kojima, Y. & Murakami, M. Screw-sense selective self-propelling polymerizations producing chiral poly(quinoxaline-2,3-diyl)s. Tetrahedron Lett. 34, 8279 (1993).
Ito, Y., Ihara, E., Murakami, M. & Sisido, M. Studies on the conformation of helical poly(2,3-quinoxaline)s.Empirical energy calculation and theoretical circular dichroism. Macromolecules 25, 6810 (1992).
Ito, Y., Kojima, Y., Suginome, M. & Murakami, M. New synthesis of quinoxaline derivatives based on palladium catalyzed oligomerization of 1-diisocyano-arenes. Heterocycles 42, 597 (1996).
Ito, Y., Kojima, Y., Murakami, M. & Suginome, M. Racemization and deracemization of poly(quinoxaline-2,3-diyl)s. Bull. Chem. Soc. Jpn. 70, 2801 (1997).
Ito, Y., Miyake, T., Hatano, S., Shima, R., Ohara, T. & Suginome, M. Asymmetric synthesis of helical poly(quinoxaline-2,3-diyl)s by palladium-mediated polymerization of 1,2-diisocyanobenzenes: Effective control of the screw-sense by a binaphthyl group at the chain-end. J. Am. Chem. Soc. 120, 11880 (1998).
Ito, Y., Ohara, T., Shima, R. & Suginome, M. Highly screw-sense selective polymerization of 1,2-diisocyano-3,6-di-p-tolylbenzene initiated by optically active binaphthylpalladium(II) complexes. J. Am. Chem. Soc. 118, 9188 (1996).
Ito, Y., Miyake, T., Ohara, T. & Suginome, M. Asymmetric synthesis of helically stable poly(quinoxaline-2,3-diyl)s having hydrophilic and/or hydrophobic side chains. Macromolecules 31, 1697 (1998).
Yamamoto, T. & Suginome, M. Helical poly(quinoxaline-2,3-diyl)s bearing metal-binding sites as polymer-based chiral ligands for asymmetric catalysis. Angew. Chem. Int. Ed. 48, 539 (2009).
Nuckolls, C., Katz, T. J., Verbiest, T., Van Elshocht, S., Kuball, H. G., Kiesewalter, S., Lovinger, A. J. & Persoons, A. Circular dichroism and UV-visible absorption spectra of the Langmuir-Blodgett films of an aggregating helicene. J. Am. Chem. Soc. 120, 8656 (1998).
Phillips, K. E. S., Katz, T. J., Jockusch, S., Lovinger, A. J. & Turro, N. J. Synthesis and properties of an aggregating heterocyclic helicene. J. Am. Chem. Soc. 123, 11899 (2001).
Haro, M., del Barrio, J., Villares, A., Oriol, L., Cea, P. & Lopez, M. C. Supramolecular architecture in Langmuir and Langmuir-Blodgett films incorporating a chiral azobenzene. Langmuir 24, 10196 (2008).
Gillgren, H., Stenstam, A., Ardhammar, M., Norden, B., Sparr, E. & Ulvenlund, S. Morphology and molecular conformation in thin films of poly-gamma-methyl-L-glutamate at the air-water interface. Langmuir 18, 462 (2002).
Acknowledgements
We thank Professor Shuji Okada and Professor Hachiro Nakanishi, IMRAM, Tohoku University, for the use of an X-ray diffractometer, and Professor Masahiko Yamaguchi, Faculty of Pharmaceutical Science, Tohoku University, for the use of CD spectrophotometer.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ito, Y., Miyake, T., Suginome, M. et al. Langmuir–Blodgett films of helical rigid-rod poly(quinoxaline-2,3-diyl)s. Polym J 42, 406–410 (2010). https://doi.org/10.1038/pj.2010.14
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pj.2010.14
Keywords
This article is cited by
-
Development of Interfacial Nanoassembly Techniques in Functional Nanomaterials
Polymer Journal (2019)