Abstract
We demonstrated that positive polyelectrolyte multilayers (PEMs) have great potential for conventional enzyme-linked immunosorbent assay (ELISA) systems because of the induction protein enrichment on their surface. In this study, we developed a novel simple molecular detection system using a PEMs-modified cellulose acetate (CA) membrane filter (PEMs-CA), which achieved rapid detection without compromising sensitivity as compared with conventional ELISA systems. This rapid detection was carried out by the centrifugal permeation of the antigen solution, thus allowing the local condensation of the antigen molecule in the proximity of the primary antibody-enriched PEMs-CA membrane, which overcame molecular diffusion as the time-limiting factor as compared with conventional ELISA systems. Hence, on the permeation system, the incubation time required for the antigen–antibody reaction corresponded to just the permeation time, which was 1/20 shorten than the conventional ELISA system under optimized conditions for the centrifugal forces. Moreover, the calibration curve of PEMs-CA had wide range of concentration from 0.02 to 5 μg ml−1 and larger change in signal as compared with the bare CA membrane. We concluded that this centrifugal permeation system should be further developed for rapid, precise and simple systems in various immunosensors using PEMs-modified membrane filters.
Similar content being viewed by others
Introduction
Since immunoassays were first developed in the 1950s, antibodies and other affinity reagents have been developed to improve these assays in terms of specificity and sensitivity.1 Moreover, the fundamental technique for using various precise immunosensors, electrochemical, optical and microgravimetric immunosensors has evolved significantly from the early devices that appeared in the late 1980s to the more sophisticated, integrated systems developed more recently.2 Economic pressures in the health-care system mean that attempts have long been made to reconcile the elements of the tension triangle: ‘fast—good—cheap’. Thus far, however, it has only been possible to satisfy only two of these elements.3, 4 Disposable immunotests (qualitative and quantitative), such as enzyme-linked immunosorbent assay (ELISA), retain the gold standard for protein detection and quantification. However, long incubation times are required to reach complete antigen–antibody reactions due to the long diffusion times of the antigen molecules toward the antibody as the rate-limiting step in these systems, resulting in slow binding kinetics and compromised assay sensitivity and dynamic range.5, 6, 7, 8
In this study, we developed a rapid, precise and simple antigen permeation immunoassay based on the positive polyelectrolyte multilayers (PEMs)-modified membrane filter (cellulose acetate, CA), which overcame the diffusion-limited reaction in conventional ELISA systems (Figure 1). We demonstrated that these PEMs can induce selective protein adsorption by electrostatic interactions between the protein and PEMs surface.9, 10, 11 Moreover, in conventional ELISA systems, positive PEMs composed of poly(diallyldimethylammonium chloride) (PDDA)/poly(sodium 4–styrenesulfonate) (PSS) have higher sensitivity as compared with conventional polystyrene plates because of a surface enrichment of the blocking reagent (coverage: 100%).12 In the present study, the primary antibody and blocking reagent were adsorbed onto the filter, set into a filter holder and then connected to a syringe containing the antigen solution to induce antibody–antigen reaction by a simple-centrifugal permeation process (Figure 1b). This detection system with high performance biosensors had several favorable characteristics as follows: (1) the PEM-modified filter can induce a three-dimensional enrichment of the primary antibody (Figure 1b; scanning electron microscope) and blocking reagent in the porous membrane, leading to higher sensitivity for recognizing antigen molecules; (2) a rapid antigen–antibody reaction is carried out by antigen molecules condensing around the primary antibody while the antigen molecules are passively permeated through the filter by centrifugal force (0.42 N, 800 r.p.m.; Figure 1b); hence, the incubation time for the antigen–antibody reaction corresponded to just the permeation time, which was 1/20 shorten than that of conventional ELISA systems (37 °C 1 h) and (3) this rapid and precise centrifugal permeation system can be applied to various molecular diagnostic biosensors because of its simple, reliable and disposable process, which improves other approaches for permeation detection in existence.13, 14, 15, 16, 17 Moreover, we considered that this permeation detection system can achieve the separation and detection of antigen molecules from whole blood simultaneously, just when the molecular cutoff of the filter is appropriate to the molecular weight of the antigen.
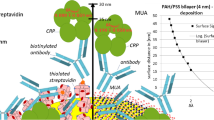
(a) Illustration of the enzyme-linked immunosorbent assay system using the permeation detection on polyelectrolyte multilayers (PEMs)-modified membrane filter (PEMs-CA). (b) Illustration of antigen detection on the PEMs-CA filter by centrifugal permeation. A scanning electron microscope image of an anti-mouse immunoglobulin G (IgG) labeled with gold colloids adsorbed on the PEMs-CA is shown. The IgG was visualized by a Silver-Enhancer reagent. The silver beads are 100- to 200-nm granules present on the surface. CA, cellulose acetate; OVA, ovalbumin.
Materials and methods
Materials
PDDA (#17338; Polyscience Inc., PA, USA; Mw=2.4 × 105 g mol−1) and PSS (#561959; Aldrich, St Louis, MO, USA; Mw=2.0 × 105 g mol−1) were used as polyelectrolytes. The diameter and pore size of the CA membrane filter (CA, C020A013A; Advantec Toyo Roshi Kaisha, Ltd, Tokyo, Japan) were 13 mm and 200 nm, respectively. Rabbit anti-mouse immunoglobulin G (IgG; M7023), mouse IgG (I5381), goat anti-mouse IgG-horseradish peroxidase conjugated IgG (goat anti-mouse IgG-HRP; A4416), ovalbumin from chicken egg white (OVA, A5503) and Silver-Enhancer Kit (SE-100) were all purchased from SIGMA (St Louis, MO, USA). Recombinant human C-reactive protein (CRP, 45104807) was purchased from Oriental Yeast Co., Ltd, Tokyo, Japan. Tris(hydroxymethyl)aminomethane hydrochloride (Tris-HCl; Wako Pure Chemical Industries, Ltd, Osaka, Japan) was used as a buffer solution. All chemicals were used without further purification. Ultrapure water was used throughout this experiment.
Fabrication of PEMs on CA filter
The concentrations of the PDDA and PSS solutions were adjusted to 0.2 mg ml−1 by 50 mmol l−1 Tris-HCl containing 0.15 mol l−1 NaCl. At first, the CA filter was immersed into the PDDA solution for 2 min at room temperature; subsequently, the filter was rinsed with 1 mmol l−1 Tris-HCl (pH 7.4) for 30 s. Next, the filter was immersed into PSS solution as described above. This alternate adsorption process was repeated for 3.5 cycles for the preparation of the positive 7-step (PDDA/PSS)3PDDA PEMs on the filter.
ELISA protocol of centrifugal permeation system
The primary antibody (rabbit anti-mouse IgG; 58 μg ml−1) and OVA (5 mg ml−1) were adsorbed onto the filters at room temperature for 2 h and at 37°C for 1 h, respectively. Thereafter, the plates were rinsed, and then the adsorbed proteins were removed by 1 wt% of n-SDS (Wako Pure Chemical Industries, Ltd). The recovered protein was evaluated using a Micro BCA kit (Pierce, No. 23235, Rockford, IL, USA) at 570 nm using a multi-well plate reader (Model 680, BIO-RAD Co. Ltd, Berkeley, CA, USA).
For the permeation system, the primary antibody and blocking reagent (OVA)-adsorbed filter was set in a filter holder, and was connected with a 1 ml syringe containing 0.5 ml of antigen solution (mouse IgG; Figure 1b). The syringe containing the antigen solution can be tightly fitted in centrifuge tube by closing the cap. Next, the antigen solution was permeated through the filter by centrifugation (5922, KUBOTA Corp., Tokyo, Japan) at 25 °C to induce the antigen–antibody reaction. The angle between the axis of rotation and the centrifuge tube was constant (the centrifuge rotor was a RA-410M2). The concentration of antigen solution was 5 μg ml−1 in the experiment of the optimization of the centrifugal permeation condition. For obtaining the calibration curve of antigen detection, the concentration of antigen solution was changed from 0.02 to 5 μg ml−1. Finally, the specific signal was detected using 1 μg ml−1 of secondary antibody (the optimized concentration) at 37°C for 1 h, and was visualized by the reaction of o-phenylenediamine hydrochloride (ML-1120T, Sumitomo Bakelite Co. Ltd, Tokyo, Japan) and horseradish peroxidase. The resulting absorbance was measured by multiplate reader at 450 nm. The noise was measured by the value of the nonspecific adsorption from secondary antibody, excluding antigen–antibody reaction process. The values of specific signal (S; the signal of antigen-secondary antibody reaction) were obtained by subtracting the noise from the signal.
Results and Discussion
Protein enrichment on the PEM-modified filter
PEMs assembly can easily modify the given substrate by controlling the structure at the nanometer level using layer-by-layer technology.18 PEMs can be stable under ambient and physiological conditions, and hence, have excellent potential in various biomaterial applications.19 A number of studies have demonstrated that PEMs exhibit versatile functions with regard to protein adsorption driven by electrostatic forces, hydrogen bonding and hydrophobic interactions, and can contribute to a higher sensitivity of the immunoassay in recent studies.12, 16, 20, 21 We investigated the protein adsorption behaviors of ELISA systems, and the relationship between the detection sensitivity and the number of layers of the PDDA/PSS PEMs as compared with conventional polystyrene plates.12 From the viewpoint of the uniformity of the PEMs surface and the signal to noise ratio, the positive 7-step (PDDA/PSS)3PDDA PEMs was an optimized multilayer in the previous study. Therefore, we concluded that the (PDDA/PSS)3PDDA PEMs-modified membrane filter (PEMs-CA) can contribute to the development of a novel permeation biosensor while maintaining the inherently high performance of positive PEMs for rapid and precise molecular detection.
The thickness of the (PDDA/PSS)3PDDA PEMs was ∼8 nm, as calculated by quartz crystal microbalance.9 Therefore, we considered that (PDDA/PSS)3PDDA could be coated onto filters with a pore size of 200 nm. Table 1 shows the amount of primary antibody and blocking reagent (ovalbumin from egg white: OVA) adsorption, and their coverage on the CA and PEMs-CA filters. The amounts of both proteins adsorbed onto the PEMs-CA were ∼1.5-fold greater than that on the CA by electrostatic interactions between the protein and PEMs surface. The isoelectric points of IgG and OVA are 6.4–9.0 and 4.9, respectively. Moreover, the local charge of the IgG molecules due to the dipole points from the Fc and (Fab)2 fragments can also contribute to the adsorption and orientation on the charged surface.24 The protein coverage was well over 100% when the dimensions were calculated by the apparent area, and hence, the protein should adsorb into the filter pore. From the scanning electron microscope images (Figure 1b, see Supplementary Figure S1), gold colloid labeled anti-mouse IgG (primary antibody) was adsorbed into the pore of the PEMs-CA, and the amount of IgG adsorption on the PEMs-CA was obviously greater than that of CA, which should be considerable in that the PEMs completely covers the inner surface of the filter pore. Therefore, the PEMs-CA could achieve high-performance immunoassay because of the high density of the primary antibody and OVA adsorption to efficiently recognize the antigen molecules (specific signal), and to suppress nonspecific adsorption from the secondary antibody (noise). Moreover, we investigated the recovery of different molecular weights of fluorescein isothiocyanate-labeled dextran through the filter (see Supplementary Figure S2). The recoveries of all sizes of fluorescein isothiocyanate-labeled dextran (70, 150 and 500 kDa) were over 97% regardless of whether the CA or PEMs-CA filter was investigated. Therefore, one can consider that similar molecular weights of protein can be permeated through the filter.
Rapid detection by centrifugal permeation system on PEMs-CA
The number of bound molecules will scale linearly with the concentration of targets in solution, and will increase linearly with time:

where kon denotes the association rate, c0 is the target concentration, X represents the fraction of reacted capture probes and Γ denotes the concentration of capture probes. With a certain lower detection threshold of the sensor for a density of bound labels (Γlim), the time required to obtain a significant signal (tlim) will scale inversely proportional to the target concentration:

To increase the sensitivity without sacrificing reaction time, one must either introduce an amplification step or a condensing step for the target or for the capture probes. Here, we created a technology that was based on amplification of the primary antibody on and/or in the filter by PEMs modification, as well as condensation of the antigen molecules by applying a centrifugal force through the antigen solution and PEMs-modified porous membrane in order to create a higher sensitivity and rapid immunoassay.
At first, we optimized the reaction conditions of the centrifugal permeation, which considered the effect of different rotational times and speeds on the specific signal to noise ratio (S/N; Figure 2). The values of specific signal (S; the signal of antigen-secondary antibody reaction) were obtained by subtracting the noise from the signal. The noise was measured by the value of the nonspecific adsorption from secondary antibody, excluding antigen–antibody reaction process. As shown in Figure 1b, the primary antibody and blocking reagent-adsorbed filter were set in the filter holder, and then it was connected with a syringe containing 0.5 ml of antigen solution to induce the antibody–antigen reaction by centrifugal permeation. The antigen solution completely permeated the filter excluding the condition of 500 r.p.m. and a 3 min duration. The antigen molecules were mostly subjected to the centrifugal force to permeate the filter, and any other forces due to gravity would be very small and can be ignored. The different rotational speeds and thus centrifugal force can induce different permeation speeds of the antigen molecules, as well as the frictional force between the antigen and membrane filter. The rotational times can induce complete permeation of the antigen solution. Figure 2 shows that the S/N value was the highest and s.d. was the smallest at 800 r.p.m. (0.42 N) and 3 min, regardless of whether the CA or PEMs-CA filter was used. The values of the specific signal and noise were added in the Supplementary Table S1. We considered that using these optimized reaction conditions for centrifugal permeation, the antigen condensation reached an equilibrium for the antigen-primary antibody reaction on or in the filter, and this should minimize any nonspecific protein adsorption and protein desorption. In the case of the PEMs-CA filter, at 800 r.p.m. and 1100 r.p.m., the S/N value decreased and increased with increasing spin times, respectively, which could be ascribed to primary antibody and/or blocking reagent desorption when the antigen solution was permeated (see Supplementary Figure S3). Moreover, we cleared that the nonspecific adsorption of the antigen did not increase by additional centrifugal force (see Supplementary Figure S4).
Figure 3a shows the specific signals with the change in antigen concentration by the centrifugal permeation system (800 r.p.m., 3 min). On the PEMs-CA filter, the s.d.s of the signals were small, moreover, a larger slope for the calibration curve was obtained (the change in signal was larger) as compared with the CA filter from 0.02 to 5 μg ml−1. This result demonstrated that the positive PEMs-modified filter can achieve a precise and rapid permeation immunoassay because of the higher enrichment of the primary antibody and blocking reagent on and/or in the filter. In contrast, using the conventional static method for a 3 min reaction, the s.d.s of the signals were large, resulting in the inability to precisely detect the antigen molecules over the same concentration range above (Figure 3b). Moreover, ‘CA- Static 3 min’ almost did not have signal change over the full range of the antigen concentration due to that of less amount of primary antibody adsorption than onto the PEMs-CA. The calibration curve for conventional ELISA detection (37 °C, 1 h) using both PEMs-CA and CA filters is shown in Figure 3c. As shown in Supplementary Figure S5, the calibration curve for centrifugal permeation detection at 500 r.p.m. and 6 min using the PEMs-CA filter (the S/N value was secondary high in Figure 2b) cannot reach the same precision as compared with 800 r.p.m. at 3 min. Therefore, the reaction conditions of 800 r.p.m. and 3 min were considered to represent optimized condition for both the S/N value and the calibration curve. Figure 4a shows the S/N values of the ‘Permeation 3 min’, ‘Static 3 min’ and ‘Static 60 min’ methods using the PEMs-CA filter at different antigen concentrations. Obviously, the s.d. of the ‘Static 3 min’ was much higher than ‘Permeation 3 min’ and ‘Static 60 min’. Indeed, the S/N value was below zero at some points in a 0.2 μg ml−1 antigen solution using the ‘Static 3 min’ method. However, the values of the ‘Permeation 3 min’ method were as stable and reliable as the conventional ‘Static 60 min’ method over the full range of antigen concentrations. In contrast, the S/N values of the ‘Permeation 3 min’ and ‘Static 60 min’ methods using CA filter were lower than that of PEMs-CA filter (Figure 4b). In the case of ‘CA- Static 3 min’, the S/N value was not regular and the s.d.s were high, resulting in poor detection. In Table 2, we compared the R2 and slope of the calibration curves, and the signal change in different concentration range of the antigen solution from Figure 3. The R2 of the ‘PEMs-CA- Permeation 3 min’, ‘PEMs-CA- Static 60 min’ and ‘PEMs-CA- Static 3 min’ methods were 0.99, 0.99 and 0.78, respectively. This result demonstrates that molecular detection by the centrifugal permeation system was 20-fold faster than the conventional ELISA system, and moreover, it accomplished this without compromising the sensitivity. In contrast, if the conventional ELISA method using the PEMs-CA filter does not proceed for a sufficiently long reaction time, then it cannot obtain precise detection because the antigen reaction is carried out by the slow diffusion of antigen molecules toward the primary antibody adsorbed onto the filter substrate. Moreover, the slope of the calibration curve and the values of the signal change in all cases of the PEMs-CA filter were higher than the bare CA membrane, which indicated that PEMs-CA can maintain the high performance of immunoassay based on positive PEMs.
The specific signal with a change in the antigen on both cellulose acetate (CA) and polyelectrolyte multilayers (PEMs)-CA filters by the different detection methods. (a) Permeation 3 min: centrifugal permeation method at room temperature for 3 min (n=6); (b) static 3 min: static method at room temperature for 3 min (n=6) and (c) static 60 min: conventional static method at 37 °C for 60 min (n=6).
Signal to noise ratios (S/Ns) of the different concentrations of antigen for the different detection methods using polyelectrolyte multilayers (PEMs)-cellulose acetate (CA) (a) and CA (b). Permeation 3 min: centrifugal permeation method at room temperature for 3 min (n=6); static 3 min: static method at room temperature for 3 min (n=6); static 60 min: conventional static method at 37 °C for 60 min (n=6).
Moreover, we investigated the detection sensitivity of the antigen performing the effect of nonspecific binding of 1 μg ml−1 C-reactive protein was added to the permeation detection system (Figure 5). The R2 of the calibration curve was 0.93. Although C-reactive protein inhibited the antigen (mouse IgG)–antibody reaction, centrifugal permeation system can detect the specific signals with the change in antigen concentration.
Taking all of the above results together, one can deduce the configuration of the protein adsorption and the mechanism of the static and permeation systems for antigen detection on each membrane filter (Figure 6). The red arrow can be considered the direction of molecular motion. Conventional static detection was carried out by random diffusion of the antigen molecules, and hence, it needs a sufficient reaction time for the antigen to move closer to the substrate and/or heterogeneously adsorbed primary antibody to reach the maximum amount of bound protein (Γlim; Figure 6a). In this study, the centrifugal permeation system induced antigen condensation around the primary antibody in order to achieve rapid detection (Figures 6b and c). The antigen molecules moved close and were bound to the primary antibody because of the passive permeation through the membrane filter, and hence, the reaction time corresponded to just the permeation times. From the above results, the permeation detection system can achieve a 20-fold faster detection than the conventional ELISA detection method regardless of the initial antigen concentration (c0). Moreover, on the PEMs-modified CA (PEMs-CA) filters, the amount of primary antibody adsorption was greater and the orientation was better24 as compared with the bare CA filter, leading to higher sensitivity to efficiently recognize to the antigen molecules (Figure 6c).
Conclusion
We developed a novel and rapid immunoassay using a simple and reliable centrifugal permeation system based on a positive PEMs-modified membrane filter. The incubation time for antigen–antibody reaction was just 3 min, and corresponded to the permeation time of the antigen solution, which this rapid system carried out by passive condensation of the antigen molecules surrounding the primary antibody. This centrifugal permeation system was convenient, and could be applied to a diverse range of rapid, precise and disposable immunosensors.
References
Oellerich, M. Enzyme-immunoassay: a review. J. Clin. Chem. Clin. Biochem. 22, 895–904 (1984).
González Oliva, A., Cruz, H. J. & Rosa, C. C. Immunosensor for diagnostics. Sensors update 9, 283–312 (2001).
Durner, J. Clinical chemistry: challenges for analytical chemistry and the nanosciences from medicine. Angew. Chem. Int. Ed. 49, 1026–1051 (2010).
Park, J. S., Cho, M. K., Lee, E. J., Ahn, K. Y., Lee, K. E., Jung, J. H., Cho, Y. J., Han, S. S., Kim, Y. K. & Lee, J. A highly sensitive and selective diagnostic assay based on virus nanoparticles. Nat. Nanotechnol. 4, 259–264 (2009).
Sapsford, K. E., Liron, Z., Shubin, Y. & Ligler, F. S. Kinetics of antigen binding to arrays of antibodies in different sized spots. Anal. Chem. 73, 5518–5524 (2001).
Karlsson, R. Real-time competitive kinetic analysis of interactions between low-molecular-weight ligands in solution and surface-immobilized receptors. Anal. Biochem. 221, 142–151 (1994).
Stenberg, M. & Nygren, H. Kinetics of antigen-antibody reactions at solid-liquid interfaces. J. Immunol. Methods 113, 3–15 (1988).
Stenberg, M., Werthén, M., Theander, S. & Nygren, H. A diffusion limited reaction theory for a microtiter plate assay. J. Immunol. Methods 112, 23–29 (1988).
Watanabe, J., Shen, H. Y. & Akashi, M. Polyelectrolyte droplets facilitate versatile layer-by-layer coating for protein loading interface. Acta Biomater. 4, 1255–1262 (2008).
Watanabe, J., Shen, H. Y. & Akashi, M. Alternate drop coating for forming dual biointerfaces composed of polyelectrolyte multilayers. J. Mater. Sci. Mater. Med. 20, 759–765 (2009).
Shen, H. Y., Watanabe, J. & Akashi, M. Heterofunctional interfaces achieve dual protein adsorption on polyelectrolyte multilayers. Polym. J. 41, 486–491 (2009).
Shen, H. Y., Watanabe, J. & Akashi, M. Polyelectrolyte multilayers-modified polystyrene plate improves conventional immunoassay: full covering of the blocking reagent. Anal. Chem. 81, 6923–6928 (2009).
Pinon, J. M., Puygauthier-Toubas, D., Lepan, H., Marx, C., Bonhomme, A., Boulant, J., Geers, R. & Dupont, H. Rapid detection of proteins by enzyme-linked immunofiltration assay after transfer onto nitrocellulose membranes. Electrophoresis 11, 41–45 (1990).
Cheek, B. J., Steel, A. B., Torres, M. P., Yu, Y. & Yang, H. Chemiluminescence detection for hybridization assays on the flow-thru chip, a three-dimensional microchannel biochip. Anal. Chem. 73, 5777–5783 (2001).
Xu, Y. & Bao, G. A filtration-based protein microarray technique. Anal. Chem. 75, 5345–5351 (2003).
Dai, J., Baker, G. L. & Bruening, M. L. Use of porous membranes modified with polyelectrolyte multilayers as substrates for protein arrays with low nonspecific adsorption. Anal. Chem. 78, 135–140 (2006).
Van Lieshout, R. M. L., Domburg, T. V., Saalmink, M., Verbeek, R., Wimberger-Friedl, R., Dieijen-Visser, M. P. V. & Punyadeera, C. Three-dimensional flow-through protein platform. Anal. Chem. 81, 5165–5171 (2009).
Decher, G. Fuzzy nanoassemblies: toward layered polymeric multicomposites. Science 277, 1232–1237 (1997).
Tang, Z. Y., Wang, Y., Podsiadlo, P. & Kotov, N. A. Biomedical applications of layer-by-layer assembly: from biomimetics to tissue engineering. Adv. Mater. 18, 3203–3224 (2006).
Sung, W. C., Chang, C. C., Makamba, H. & Chen, S. H. Long-term affinity modification on poly(dimethylsiloxane) substrate and its application for ELISA analysis. Anal. Chem. 80, 1529–1535 (2008).
Derveaux, S., Stubbe, B. G., Roelant, C., Leblans, M., De Geest, B. G., Demeester, J. & De Smedt, S. C. Layer-by-layer coated digitally encoded microcarriers for quantification of proteins in serum and plasma. Anal. Chem. 80, 85–94 (2008).
Silverton, E. W., Navia, M. A. & Davies, D. R. Three-dimensional structure of an intact human immunoglobulin. Proc. Natl Acad. Sci. USA 74, 5140–5144 (1977).
Kamilya, T., Pal, P. & Talapatra, G. B. Interaction of ovalbumin with phospholipids langmuir-blodgett film. J. Phys. Chem. B 111, 1199–1205 (2007).
Zhou, J., Tsao, H. K., Sheng, Y. J. & Jiang, S. Y. Monte carlo simulations of antibody adsorption and orientation on charged surfaces. J. Chem. Phys. 121, 1050–1057 (2004).
Acknowledgements
We thank Drs T Kida, M Matsusaki, H Ajiro and T Akagi from Osaka University for their helpful discussions and advice. One of the authors (HS) was supported by a grant from Global Education and Research Center for Bio-Environmental Chemistry and a scholarship from Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on Polymer Journal website
Supplementary information
Rights and permissions
About this article
Cite this article
Shen, H., Watanabe, J. & Akashi, M. Polyelectrolyte multilayers-modified membrane filter for rapid immunoassay: protein condensation by centrifugal permeation. Polym J 43, 35–40 (2011). https://doi.org/10.1038/pj.2010.114
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pj.2010.114