Abstract
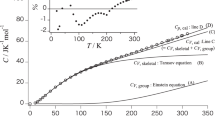
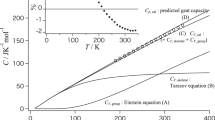
Heat capacities of poly(oxacyclobutane), subject to three kinds of thermal history, were measured in the temperature region from liquid helium temperature (1.4°K) to 330°K by using two kinds of adiabatic calorimeters and the effect of thermal treatment upon heat capacity was studied. As a result, it was found that the effect of thermal treatment was evident in the low temperature region (below 15°K) as well as around the glass transition region.Furthermore, the heat capacity of the completely crystalline state was estimated and analysed, based on Tarasov’s model, between 13 and 250°K. Characteristic temperatures θ1 and θ3 in Tarasov’s model were evaluated to be 605 and 97°K, respectively. Below 13°K, the temperature dependence of the heat capacity was examined for crystalline, semicrystalline, and glassy states.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
S. Yoshida, M. Sakiyam, and S. Seki, Polym. J., 1, 573 (1970).
W. Reese and O. Tucker, J. Chem. Phys., 46, 1388 (1967).
V. V. Tarasov, “New Problems in the Physics of Glass,” Israel Program for Scientific Translations, Jerusalem, 1963.
B. Wunderlich, J. Chem. Phys., 37, 1207 (1962).
H. Tadokoro, Y. Takahashi, Y. Chatani, and H. Kakida, Makromol. Chem., 109, 96 (1967).
J. B. Rose, J. Chem. Soc., 526 (1956).
K. Yamamoto, A. Teramoto, and H. Fujita, Polymer, 7, 267 (1966).
M. Sorai, H. Suga, and S. Seki, Bull. Chem. Soc. Jpn., 41, 312 (1968).
T. Matsuo, H. Suga, and S. Seki, J. Phys. Soc. Jpn., 30, 785 (1971).
M. Dole, J. Polym. Sci., Part C, 18, 57 (1967).
W. Nernst and F. A. Lindemann, Z. Electrochem., 17, 817 (1911).
B. Wunderlich, J. Chem. Phys., 37, 1203 (1962).
D. Makino, M. Kobayashi, and H. Tadokoro, J. Chem. Phys., 51, 3901 (1969).
H. B. Rosenstock, J. Phys. Chem. Solids, 23, 659 (1962).
P. Flubacher, A. J. Leadbetter, J. A. Morrison, and B. P. Stoicheff, J. Phys. Chem. Solids, 12, 53 (1959).
A. A. Antoniou and J. A. Morrison, J. Appl. Phys., 36, 1873 (1965).
R. S. Craig, C. W. Massena, and R. M. Mallya, J. Appl. Phys., 36, 108 (1965).
G. L. Salinger, C. L. Choy, and R. G. Hunt, J. Chem. Phys., 52, 3629 (1970).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yoshida, S., Suga, H. & Seki, S. Thermodynamic Studies of Solid Polyethers. II. Heat Capacity of Poly(oxacyclobutane), –[–(CH2)3O–]–n, between 1.4 and 330°K. Polym J 5, 11–24 (1973). https://doi.org/10.1295/polymj.5.11
Issue Date:
DOI: https://doi.org/10.1295/polymj.5.11
Keywords
This article is cited by
-
Analysis of the configurational heat capacity of polystyrene and its monomer and oligomer above the glass transition temperature
Polymer Journal (2022)
-
Configurational heat capacity of various polymers above the glass transition temperature
Polymer Journal (2022)
-
Prediction of the heat capacity of main-chain-type polymers below the glass transition temperature
Polymer Journal (2020)
-
Heat capacities of polymer solids composed of polyesters and poly(oxide)s, evaluated below the glass transition temperature
Polymer Journal (2020)