Abstract
Background:
Previously, we showed that carbohydrate restriction with calorie restriction slowed tumor growth in xenograft mouse prostate cancer models. Herein, we examined the impact of carbohydrate restriction without calorie restriction on tumor development within the context of diet-induced obesity in the Hi-Myc transgenic mouse model of prostate cancer.
Methods:
Mice were randomized at 5 weeks of age to ad libitum western diet (WD; 40% fat, 42% carbohydrate; n=39) or ad libitum no carbohydrate ketogenic diet (NCKD; 82% fat, 1% carbohydrate; n=44). At age 3 or 6 months, mice were killed, prostates weighed and prostate histology, proliferation, apoptosis and macrophage infiltration evaluated by hematoxylin and eosin, Ki67, TUNEL and F4/80 staining, respectively. Body composition was assessed by DEXA, serum cytokines measured using multiplex, and Akt/mTOR signaling assessed by Western.
Results:
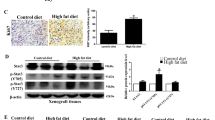
Caloric intake was higher in the NCKD group, resulting in elevated body weights at 6 months of age, relative to the WD group (45 g vs 38g; P=0.008). Despite elevated body weights, serum monocyte chemoattractant protein (MCP)-1 and interleukin (IL)-1α levels were lower in NCKD versus WD mice (P=0.046 and P=0.118, respectively), and macrophage infiltration was reduced in prostates of NCKD versus WD mice (P=0.028). Relative Akt phosphorylation and phospho-S6 ribosomal protein levels were reduced in prostates of NCKD versus WD mice. However, while mice randomized to NCKD had smaller prostates after adjustment for body weight at 3 and 6 months (P=0.004 and P=0.002, respectively), NCKD mice had higher rates of adenocarcinoma at 6 months compared to WD mice (100 vs 80%, P=0.04).
Conclusions:
Despite higher caloric intake and elevated body weights, carbohydrate restriction lowered serum MCP-1 levels, reduced prostate macrophage infiltration, reduced prostate weight, but failed to slow adenocarcinoma development. Together, these data suggest that although carbohydrate restriction within the context of obesity may reduce obesity-associated systemic inflammation and perhaps slow tumor growth, it is not sufficient to counteract obesity-associated tumor development.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Allott EH, Masko EM, Freedland SJ . Obesity and prostate cancer: weighing the evidence. Eur Urol 2013; 63: 800–809.
Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM . Prevalence of overweight and obesity in the United States, 1999–2004. J Am Med Assoc 2006; 295: 1549–1555.
Siegel R, Naishadham D, Jemal A . Cancer statistics, 2013. CA: Cancer J Clin 2013; 63: 11–30.
Blando J, Moore T, Hursting S, Jiang G, Saha A, Beltran L et al. Dietary energy balance modulates prostate cancer progression in Hi-Myc mice. Cancer Prev Res 2011; 4: 2002–2014.
Bonorden MJ, Rogozina OP, Kluczny CM, Grossmann ME, Grambsch PL, Grande JP et al. Intermittent calorie restriction delays prostate tumor detection and increases survival time in TRAMP mice. Nutr Cancer 2009; 61: 265–275.
Freedland SJ, Mavropoulos J, Wang A, Darshan M, Demark-Wahnefried W, Aronson WJ et al. Carbohydrate restriction, prostate cancer growth, and the insulin-like growth factor axis. The Prostate 2008; 68: 11–19.
Mavropoulos JC, Buschemeyer WC 3rd, Tewari AK, Rokhfeld D, Pollak M, Zhao Y et al. The effects of varying dietary carbohydrate and fat content on survival in a murine LNCaP prostate cancer xenograft model. Cancer Prev Res 2009; 2: 557–565.
Berrigan D, Lavigne JA, Perkins SN, Nagy TR, Barrett JC, Hursting SD . Phenotypic effects of calorie restriction and insulin-like growth factor-1 treatment on body composition and bone mineral density of C57BL/6 mice: implications for cancer prevention. In vivo 2005; 19: 667–674.
Ittmann M, Huang J, Radaelli E, Martin P, Signoretti S, Sullivan R et al. Animal models of human prostate cancer: the consensus report of the New York meeting of the Mouse Models of Human Cancers Consortium Prostate Pathology Committee. Cancer Res 2013; 73: 2718–2736.
Berquin IM, Min Y, Wu R, Wu J, Perry D, Cline JM et al. Modulation of prostate cancer genetic risk by omega-3 and omega-6 fatty acids. J Clin Invest 2007; 117: 1866–1875.
Zhang J, Lu Y, Pienta KJ . Multiple roles of chemokine (C-C motif) ligand 2 in promoting prostate cancer growth. J Natl Cancer Inst 2010; 102: 522–528.
Schwarzfuchs D, Golan R, Shai I . Four-year follow-up after two-year dietary interventions. N Engl J Med 2012; 367: 1373–1374.
Shai I, Schwarzfuchs D, Henkin Y, Shahar DR, Witkow S, Greenberg I et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med 2008; 359: 229–241.
Lu Y, Chen Q, Corey E, Xie W, Fan J, Mizokami A et al. Activation of MCP-1/CCR2 axis promotes prostate cancer growth in bone. Clin Exp Metastasis 2009; 26: 161–169.
Sharma J, Gray KP, Harshman LC, Evan C, Nakabayashi M, Fichorova R et al. Elevated IL-8, TNF-alpha, and MCP-1 in men with metastatic prostate cancer starting androgen-deprivation therapy (ADT) are associated with shorter time to castration-resistance and overall survival. The Prostate 2014; 74: 820–828.
Loberg RD, Ying C, Craig M, Day LL, Sargent E, Neeley C et al. Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo. Cancer Res 2007; 67: 9417–9424.
Loberg RD, Ying C, Craig M, Yan L, Snyder LA, Pienta KJ . CCL2 as an important mediator of prostate cancer growth in vivo through the regulation of macrophage infiltration. Neoplasia 2007; 9: 556–562.
Huang M, Narita S, Numakura K, Tsuruta H, Saito M, Inoue T et al. A high-fat diet enhances proliferation of prostate cancer cells and activates MCP-1/CCR2 signaling. The Prostate 2012; 72: 1779–1788.
Pienta KJ, Machiels JP, Schrijvers D, Alekseev B, Shkolnik M, Crabb SJ et al. Phase 2 study of carlumab (CNTO 888), a human monoclonal antibody against CC-chemokine ligand 2 (CCL2), in metastatic castration-resistant prostate cancer. Investig New Drugs 2013; 31: 760–768.
Forsythe CE, Phinney SD, Fernandez ML, Quann EE, Wood RJ, Bibus DM et al. Comparison of low fat and low carbohydrate diets on circulating fatty acid composition and markers of inflammation. Lipids 2008; 43: 65–77.
Kobayashi N, Barnard RJ, Said J, Hong-Gonzalez J, Corman DM, Ku M et al. Effect of low-fat diet on development of prostate cancer and Akt phosphorylation in the Hi-Myc transgenic mouse model. Cancer Res 2008; 68: 3066–3073.
Allott EH, Howard LE, Cooperberg MR, Kane CJ, Aronson WJ, Terris MK et al. Serum lipid profile and risk of prostate cancer recurrence: results from the SEARCH database. Cancer Epidemiol Biomarkers Prev 2014; 23: 2349–2356.
Acknowledgements
We wish to acknowledge the following individuals for their help and important contributions to this study: Yulin Zhao, Traci Reddick, Julie Kent, Bentley Midkiff. This work was supported by the National Cancer Institute [1-R01-CA131235-01A1 (SJ Freedland), 1K24CA160653 (SJ Freedland), 3R01CA125618 (E Macias) and 5R25-CA126938-03 (EH Allott)], the American Institute for Cancer Research (EH Allott) and The Prostate Cancer Foundation (E Macias).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Prostate Cancer and Prostatic Diseases website
Supplementary information
Rights and permissions
About this article
Cite this article
Allott, E., Macias, E., Sanders, S. et al. Impact of carbohydrate restriction in the context of obesity on prostate tumor growth in the Hi-Myc transgenic mouse model. Prostate Cancer Prostatic Dis 20, 165–171 (2017). https://doi.org/10.1038/pcan.2016.73
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2016.73
This article is cited by
-
Serum metabolomic analysis of men on a low-carbohydrate diet for biochemically recurrent prostate cancer reveals the potential role of ketogenesis to slow tumor growth: a secondary analysis of the CAPS2 diet trial
Prostate Cancer and Prostatic Diseases (2022)
-
Ketogenic diets consumed during radio-chemotherapy have beneficial effects on quality of life and metabolic health in patients with rectal cancer
European Journal of Nutrition (2022)
-
Beneficial effects of ketogenic diets for cancer patients: a realist review with focus on evidence and confirmation
Medical Oncology (2017)