Abstract
Background:
Approximately half of the prostate carcinomas are characterized by a chromosomal rearrangement fusing the androgen-regulated gene TMPRSS2 to the oncogenic ETS transcription factor ERG. Aim of this study was to comprehensively analyze the role and impact of the ERG rearrangement and protein expression on the progression to castration-resistant (CR) disease.
Methods:
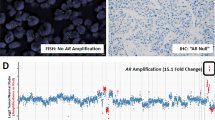
We used a tissue microarray (TMA) constructed from 114 hormone naive (HN) and 117 CR PCs. We analyzed the ERG rearrangement status by fluorescence in situ hybridization and the expression profiles of ERG, androgen receptor (AR) and the proliferation marker Ki67 by immunohistochemistry.
Results:
Nearly half of the PC tissue specimens (HN: 38%, CR: 46%) harbored a TMPRSS2-ERG gene fusion. HN PCs with positive translocation status showed increased tumor cell proliferation (P<0.05). As expected, TMPRSS2-ERG gene fusion was strongly associated with increased ERG protein expression in HN and CR PCs (both P<0.0001). Remarkably, the study revealed a subgroup (26%) of CR PCs with ERG rearrangement but without any detectable ERG protein expression. This subgroup showed significantly lower levels of AR protein expression and androgen-regulated serum PSA (both P<0.05).
Conclusions:
In this study, we identified a subgroup of ERG-rearranged CR PCs without detectable ERG protein expression. Our results suggest that this subgroup could represent CR PCs with a dispensed AR pathway. These tumors might represent a thus far unrecognized subset of patients with AR-independent CR PC who may not benefit from conventional therapy directed against the AR pathway.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Siegel R, Naishadham D, Jemal A . Cancer statistics, 2013. CA Cancer J Clin 2013; 63: 11–30.
Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 2005; 310: 644–648.
Kumar-Sinha C, Tomlins SA, Chinnaiyan AM . Recurrent gene fusions in prostate cancer. Nat Rev Cancer 2008; 8: 497–511.
Svensson MA, LaFargue CJ, MacDonald TY, Pflueger D, Kitabayashi N, Santa-Cruz AM et al. Testing mutual exclusivity of ETS rearranged prostate cancer. Lab Invest 2011; 91: 404–412.
Rubin MA, Maher CA, Chinnaiyan AM . Common gene rearrangements in prostate cancer. J Clin Oncol 2011; 29: 3659–3668.
Tomlins SA, Bjartell A, Chinnaiyan AM, Jenster G, Nam RK, Rubin MA et al. ETS gene fusions in prostate cancer: from discovery to daily clinical practice. Eur Urol 2009; 56: 275–286.
Perner S, Demichelis F, Beroukhim R, Schmidt FH, Mosquera JM, Setlur S et al. TMPRSS2:ERG fusion-associated deletions provide insight into the heterogeneity of prostate cancer. Cancer Res 2006; 66: 8337–8341.
Sreenath TL, Dobi A, Petrovics G, Srivastava S . Oncogenic activation of ERG: a predominant mechanism in prostate cancer. J Carcinog 2011; 10: 37.
Clark JP, Cooper CS . ETS gene fusions in prostate cancer. Nat Rev Urol 2009; 6: 429–439.
Rosen P, Sesterhenn IA, Brassell SA, McLeod DG, Srivastava S, Dobi A et al. Clinical potential of the ERG oncoprotein in prostate cancer. Nat Rev Urol 2012; 9: 131–137.
Minner S, Enodien M, Sirma H, Luebke AM, Krohn A, Mayer PS et al. ERG status is unrelated to PSA recurrence in radically operated prostate cancer in the absence of antihormonal therapy. Clin Cancer Res 2011; 17: 5878–5888.
Rickman DS, Chen YB, Banerjee S, Pan Y, Yu J, Vuong T et al. ERG cooperates with androgen receptor in regulating trefoil factor 3 in prostate cancer disease progression. Neoplasia 2010; 12: 1031–1040.
Scheble VJ, Scharf G, Braun M, Ruiz C, Stürm S, Petersen K et al. ERG rearrangement in local recurrences compared to distant metastases of castration-resistant prostate cancer. Virchows Arch 2012; 461: 157–162.
Hermans KG, van Marion R, van Dekken H, Jenster G, van Weerden WM, Trapman J et al. TMPRSS2:ERG fusion by translocation or interstitial deletion is highly relevant in androgen-dependent prostate cancer, but is bypassed in late-stage androgen receptor-negative prostate cancer. Cancer Res 2006; 66: 10658–10663.
Cai C, Wang H, Xu Y, Chen S, Balk SP . Reactivation of androgen receptor-regulated TMPRSS2:ERG gene expression in castration-resistant prostate cancer. Cancer Res 2009; 69: 6027–6032.
Mehra R, Tomlins SA, Yu J, Cao X, Wang L, Menon A et al. Characterization of TMPRSS2-ETS gene aberrations in androgen-independent metastatic prostate cancer. Cancer Res 2008; 68: 3584–3590.
Kononen J, Bubendorf L, Kallioniemi A, Bärlund M, Schraml P, Leighton S et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 1998; 4: 844–847.
Zellweger T, Stürm S, Rey S, Zlobec I, Gsponer JR, Rentsch CA et al. Estrogen receptor β expression and androgen receptor phosphorylation correlate with a poor clinical outcome in hormone-naive prostate cancer and are elevated in castration-resistant disease. Endocr Relat Cancer 2013; 20: 403–413.
Furusato B, Tan SH, Young D, Dobi A, Sun C, Mohamed AA et al. ERG oncoprotein expression in prostate cancer: clonal progression of ERG-positive tumor cells and potential for ERG-based stratification. Prostate Cancer Prostatic Dis 2010; 13: 228–237.
Miettinen M, Wang ZF, Paetau A, Tan SH, Dobi A, Srivastava S et al. ERG transcription factor as an immunohistochemical marker for vascular endothelial tumors and prostatic carcinoma. Am J Surg Pathol 2011; 35: 432–441.
R Core Team (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria. http://www.R-project.org.
Hothorn T, Hornik K, van de Wiel MA, Zeileis A . Implementing a class of permutation tests: the coin package. American Statis 2007; 60: 257–263.
Petrovics G, Liu A, Shaheduzzaman S, Furusato B, Sun C, Chen Y et al. Frequent overexpression of ETS-related gene-1 (ERG1) in prostate cancer transcriptome. Oncogene 2005; 24: 3847–3852.
Park K, Liu A, Shaheduzzaman S, Furusato B, Sun C, Chen Y et al. Antibody-based detection of ERG rearrangement-positive prostate cancer. Neoplasia 2010; 12: 590–598.
Braun M, Goltz D, Shaikhibrahim Z, Vogel W, Böhm D, Scheble V et al. ERG protein expression and genomic rearrangement status in primary and metastatic prostate cancer—a comparative study of two monoclonal antibodies. Prostate Cancer Prostatic Dis 2012; 15: 165–169.
Mosquera JM, Mehra R, Regan MM, Perner S, Genega EM, Bueti G et al. Prevalence of TMPRSS2-ERG fusion prostate cancer among men undergoing prostate biopsy in the United States. Clin Cancer Res 2009; 15: 4706–4711.
Tu JJ, Rohan S, Kao J, Kitabayashi N, Mathew S, Chen YT et al. Gene fusions between TMPRSS2 and ETS family genes in prostate cancer: frequency and transcript variant analysis by RT-PCR and FISH on paraffin-embedded tissues. Mod Pathol 2007; 20: 921–928.
St John J, Powell K, LaComb MKC . TMPRSS2-ERG fusion gene expression in prostate tumor cells and its clinical and biological significance in prostate cancer progression. JCST 2012; 04.
Teng L-H, Wang C, Bégin LR, Dolph M, Yilmaz A, Trpkov K et al. ERG protein expression and gene rearrangements are present at lower rates in metastatic and locally advanced castration-resistant prostate cancer compared to localized disease. Urology 2013; 82: 394–399.
Fine SW, Gopalan A, Leversha MA, Al-Ahmadie HA, Tickoo SK, Zhou Q et al. TMPRSS2-ERG gene fusion is associated with low Gleason scores and not with high-grade morphological features. Mod Pathol 2010; 23: 1325–1333.
Dobi A, Furusato B, Shaheduzzaman S, Chen Y, Vahey M, Nydam T et al. ERG expression levels in prostate tumors reflect functional status of the androgen receptor (AR) as a consequence of fusion of ERG with AR regulated gene promoters. Open Can Jour 2010; 3: 101–108.
Mostaghel EA, Geng L, Holcomb I, Coleman IM, Lucas J, True LD et al. Variability in the androgen response of prostate epithelium to 5alpha-reductase inhibition: implications for prostate cancer chemoprevention. Cancer Res 2010; 70: 1286–1295.
Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ et al. Autonomic nerve development contributes to prostate cancer progression. Science 2013; 341: 1236361.
Acknowledgements
This work was supported by the Krebsforschung Schweiz (KFS-02780-02-2011) to CR, by the German Research Foundation (Deutsche Forschungsgemeinschaft (DFG), Emmy-Noether-Program, PE1179/2-1), the Rudolf-Becker-Foundation and the Wilhelm Sander-Foundation (No. 2011.077.1) to SP, by the RO1 DK065977 to SS and by the DoD, CDMRP, PC073614 to SS and AD. We thank Thuy Nguyen and Petra Hirschmann for excellent technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The Henry M. Jackson Foundation for the Advancement of Military Medicine has filed a patent application on the mouse monoclonal anti-ERG antibody, 9FY, on which ST, AD and SS are co-inventors and has been licensed to the Biocare Medical. This study was conducted independent of any involvement from Biocare Medical. The Brigham and Women’s Hospital and the University of Michigan have filed a patent on ETS gene rearrangements in prostate cancer, on which SP is a co-inventor and the diagnostic field of use has been licensed to GenProbe. GenProbe has not played a role in the design and conduct of the study, in the collection, analysis or interpretation of the data and had no involvement in the preparation, review or approval of the manuscript. All the other authors declare no conflict of interest.
Additional information
Supplementary Information accompanies the paper on the Prostate Cancer and Prostatic Diseases website
Rights and permissions
About this article
Cite this article
Gsponer, J., Braun, M., Scheble, V. et al. ERG rearrangement and protein expression in the progression to castration-resistant prostate cancer. Prostate Cancer Prostatic Dis 17, 126–131 (2014). https://doi.org/10.1038/pcan.2013.62
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2013.62
Keywords
This article is cited by
-
Prostatic adenocarcinoma CNS parenchymal and dural metastases: alterations in ERG, CHD1 and MAP3K7 expression
Journal of Neuro-Oncology (2019)
-
Ethnicity and ERG frequency in prostate cancer
Nature Reviews Urology (2018)