Abstract
Background:
Intermittent androgen deprivation (IAD) for prostate cancer was studied with the objective of reducing the side effects of treatment and potentially delaying the development of hormone resistance. There also appears to be a quality of life benefit during off-treatment intervals owing to the recovery of serum testosterone levels.
Methods:
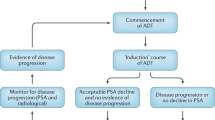
In this multicentre European prospective randomised phase III trial EC507, testosterone serum concentrations were analysed in prostate cancer patients with PSA progression after radical prostatectomy. Patients were randomised to a continuous androgen deprivation (CAD) and IAD therapy using a 3-month depot with 11.25 mg leuprorelin acetate as microcapsule formulation. A complete IAD cycle comprises both a 6-month androgen deprivation therapy plus the off-treatment time (OTT).
Results:
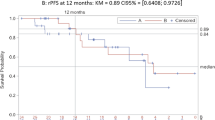
Serum testosterone recovery was recorded in 109 patients during OTT in the IAD group. Testosterone recovery to baseline values was achieved in 79.3% during the first and in 64.9% during the second IAD cycle, respectively. Median time to testosterone normalisation was 100 days in the first and 115 days in the second cycle, respectively. No significant difference was observed up to 1000 days between IAD and CAD with regard to time to androgen-independent progression. This is the first prospective study of leuprorelin acetate 11.25 mg demonstrating normalisation of testosterone levels in the off-treatment period in patients undergoing IAD.
Conclusions:
The prerequisite of an IAD treatment is the testosterone recovery during off-treatment periods. In this study, in patients with PSA relapse after radical prostatectomy, a real achievement of intermittent castration with normalisation of testosterone levels during off-treatment periods could be confirmed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Heidenreich A, Bolla M, Joniau S, Van der Kwast JH, Matveev VB, Mason MD et al. Guidelines on prostate cancer. Eur Assoc Urol 2010. http://www.uroweb.org/gls/pdf/Prostate%20Cancer%202010%20June%2017th.pdf.
Akakura K, Bruchovsky N, Goldenberg SL, Rennie PS, Buckley AR, Sullivan LD . Effects of intermittent androgen suppression on androgen-dependent tumors. Apoptosis and serum prostate-specific antigen. Cancer 1993; 71: 2782–2790.
Wright JL, Higano CS, Lin DW . Intermittent androgen deprivation: clinical experience and practical applications. Urol Clin North Am 2006; 33: 167–179.
Boccon-Gibod L, Hammerer P, Madersbacher S, Mottet N, Prayer-Galetti T, Tunn U . The role of intermittent androgen deprivation in prostate cancer. BJU Int 2007; 100: 738–743.
Spry NA, Galvão DA, Davies R, La Bianca S, Joseph D, Davidson A et al. Long-term effects of intermittent androgen suppression on testosterone recovery and bone mineral density: results of a 33-month observational study. BJU Int 2009; 104: 806–812.
Calais da Silva FC, Bono AV, Whelan P, Brausi M, Queimadelos AM, Martin JAP et al. Intermittent androgen deprivation for locally advanced and metastatic prostate cancer: Results from a randomised phase III study of the South European Uroncological Group. Eur Urol 2009; 55: 1269–1277.
German Society of Urology. Interdisciplinary S3 guideline of quality for early detection, diagnosis and treatment of various stages of prostate cancer. 2009. http://www.uro-freiburg.de/S3-Leitlinie-Prostatakarzinom-2009,102.html.
Bong GW, Clarke Jr HS, Hancock WC, Keane TE . Serum testosterone recovery after cessation of long-term luteinizing hormone-releasing hormone agonist in patients with prostate cancer. Urology 2007; 71: 1177–1180.
Gulley JL, Figg WD, Steinberg SM, Carter J, Hussain MH, Dahut WL . A prospective analysis of the time to normalization of serum androgens following 6 months of androgen deprivation therapy in patients on a randomized phase III clinical trial using limited hormonal therapy. J Urol 2005; 173: 1567–1571.
Gulley JL, Aragon-Ching JB, Steinberg SM, Hussain MH, Sartor O, Higano CS et al. Kinetics of serum androgen normalization and factors associated with testosterone reserve after limited androgen deprivation therapy for non-metastatic prostate cancer. J Urol 2008; 180: 1432–1436.
Gleave M, Klotz L, Taneja SS . The continued debate: intermittent vs continuous hormonal ablation for metastatic prostate cancer. Urol Oncol 2009; 27: 81–86.
Tunn UW . Can intermittent hormone therapy fulfill its promise? Eur Urol 2008; (Suppl 7): 752–757.
Tunn UW, Canepa G, Hillger H, Fuchs W . Intermittent androgen deprivation in patients with PSA relapse after radical prostatectomy-final result of a european randomized prospective phase-III clinical trial AUO study AP 06/95, ED 507. J Urol 2007; 177: 201 (abstr. 600).
Tunn UW . The current status of intermittent androgen deprivation (IAD) therapy for prostate cancer: putting IAD under the spotlight. BJU Int 2007; 99: 19–22.
Bostwick DG . Staging prostate cancer—1997: current methods and limitations. Eur Urol 1997; 32 (Suppl 3): 2–14.
Tunn UW . Intermittent endocrine therapy of prostate cancer. Eur Urol 1996; 30 (Suppl 1): 22–25.
Figg WD, Dahut W, Duray P, Hamilton M, Tompkins A, Steinber SM et al. A randomized phase II trial of thalidomide an angiogenesis inhibitor in patients with androgen-independent prostate cancer. Clin Cancer Res 2001; 7: 1888–1983.
Figg WD, Hussain MH, Gulley JL, Arien PM, Aragon-Ching JB, Petrylak DP et al. A double-blind randomized crossover study of oral Thalidomide versus placebo for androgen dependent prostate cancer treated with intermittent androgen ablation. J Urol 2009; 181: 1104–1113.
Oefelein M . Serum testosterone-based luteinizing hormone-releasing hormone agonist redosing schedule for chronic androgen ablation: a phase I assessment. Urology 1999; 54: 694–699.
Spry NA, Kristjanson L, Hooton B, Hayden L, Neerhut G, Gurney H et al. Adverse effects to quality of life arising from treatment can recover with intermittent androgen suppression in men with prostate cancer. Eur J Cancer 2006; 42: 1083–1092.
Klotz L, O’Callaghan CJ, Ding K, Dearnaley DP, Higano CS, Horwitz EM et al. A phase III randomized trial comparing intermittent versus continuous androgen suppression for patients with PSA progression after radical therapy: NCIC CTG PR.7/SWOG JPR.7/CTSU JPR.7/UK Intercontinental Trial CRUKE/01/013. J Clin Oncol 2011; 29 (Suppl 7): (abstr 3).
Acknowledgements
This study was sponsored by Takeda Pharma GmbH Germany and Takeda Italia Farmaceutici SPA.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Professor Ulf Tunn is consultant and lecturer for Abbott, Astellas, Bayer Health, Novartis, Sanofi-Aventis and Takeda. Dr Giorgio Canepa and Dr Andreas Kochanowsky declare no potential conflict of interest. Dr Erika Kienle is consultant and lecturer for Abbott and Takeda.
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Tunn, U., Canepa, G., Kochanowsky, A. et al. Testosterone recovery in the off-treatment time in prostate cancer patients undergoing intermittent androgen deprivation therapy. Prostate Cancer Prostatic Dis 15, 296–302 (2012). https://doi.org/10.1038/pcan.2012.12
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2012.12
Keywords
This article is cited by
-
The Role of Intermittent Androgen Deprivation Therapy in the Management of Biochemically Recurrent or Metastatic Prostate Cancer
Current Urology Reports (2015)
-
Intermittent versus continuous androgen deprivation for locally advanced, recurrent or metastatic prostate cancer: a systematic review and meta-analysis
BMC Urology (2014)
-
Intermittent androgen deprivation is a rational standard-of-care treatment for all stages of progressive prostate cancer: results from a systematic review and meta-analysis
Prostate Cancer and Prostatic Diseases (2014)
-
Intermittent versus continuous cyproterone acetate in bone metastatic prostate cancer: results of a randomized trial
World Journal of Urology (2014)