Abstract
Background:
In the era when various treatment agents for advanced-stage prostate cancer are available, it is important to investigate overall survival for metastatic prostate cancer treated with step-up hormonal treatment as a reference against new treatment regimens, including antitumor agents and/or new hormonal derivatives, and it is desirable to explore pretreatment, biopsy-related and post-treatment prognostic factors to establish tailor-made treatment strategies.
Methods:
Between 1992 and 2002, 94 patients were diagnosed with prostate cancer with distant metastases in our facility. Various pretreatment clinical findings including serum PSA, testosterone, alkaline phosphatase (ALP), digital rectal examination (DRE) and extension of disease score were investigated for predicting outcomes in step-up hormonal treatment. We also investigated the impact of pathological findings including Gleason grading, tumor volume indices, various Gleason grade 5 volume indices in biopsy specimens, and post-treatment PSA nadir following step-up hormonal treatment on overall survival.
Results:
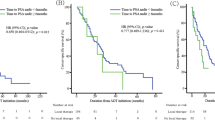
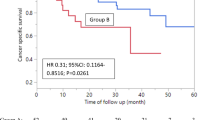
The 3- and 5-year overall survival was 72.4% and 62.5%, respectively. According to univariate analyses, patients with PSA ⩽100 ng ml–1, ALP <440 IU l–1, T1c to T3 on DRE, extension of disease (EOD) score 0–3, no Gleason grade 5 cancer in biopsy specimens or less such cancer and good response at any stage of hormonal therapy had significantly better overall survival than did patients with alternative status. A multivariate Cox proportional hazard model revealed that PSA nadir after first-line hormonal treatment, PSA nadir after second-line treatment and Gleason grade 5 volume index were independent prognostic factors.
Conclusions:
Even in very advanced prostate cancer, local pathological indices, a Gleason grade 5 volume index in particular, could differentiate patients with better prognosis from worse prognosis. Step-up hormonal therapy including luteinizing hormone-releasing hormone agonist, estrogen derivatives and steroid hormones may be valuable in patients with metastatic prostate cancer, especially in good responders at any stage of hormonal therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bartsch G, Horninger W, Klocker H, Pelzer A, Bektic J, Oberaigner W et al. Tyrol Prostate Cancer Demonstration Project: early detection, treatment, outcome, incidence and mortality. BJU Int 2008; 101: 809–816.
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T et al. Cancer statistics, 2008. CA Cancer J Clin 2008; 58: 71–96.
Ito K, Yamamoto T, Takechi H, Suzuki K . Impact of exposure rate of PSA-screening on clinical stage of prostate cancer in Japan. J Urol 2006; 175 (Suppl): 477–478.
Cancer statistics in Japan 2010. Number of Deaths, by Cancer Site (2008/2009).
Cancer Registration Committee of the Japanese Urological Association. Clinicopathological statistics on registered prostate cancer patients in Japan: 2000 report from the Japanese Urological Association. Int J Urol 2005; 12: 46–61.
Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004; 351: 1502–1512.
Petrylak DP, Tangen CM, Hussain MH, Lara Jr PN, Jones JA, Taplin ME et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med 2004; 351: 1513–1520.
Scher HI, Beer TM, Higano CS, Anand A, Taplin ME, Efstathiou E et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet 2010; 375: 1437–1446.
de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med 2011; 364: 1995–2005.
Dreicer R, Agus DB, MacVicar GR, MacLean D, Zhang T, Stadler WM . Safety, pharmacokinetics, and efficacy of TAK-700 in castration-resistant, metastatic prostate cancer: a phase I/II, open-label study. American Society of Clinical Oncology Genitourinary Cancers Symposium (ASCO GU). San Francisco, CA, March 5–7, 2010.
Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 2010; 363: 411–422.
Antonarakis ES, Eisenberger MA . Expanding treatment options for metastatic prostate cancer. N Engl J Med 2011; 364: 2055–2058.
Soloway MS, Hardeman SW, Hickey D, Raymond J, Todd B, Soloway S et al. Stratification of patients with metastatic prostate cancer based on extent of disease on initial bone scan. Cancer 1988; 61: 195–202.
Epstein JI, Allsbrook WC, Amin MB, Egevad LL . The 2005 international society of urological pathology (ISUP) consensus conference on Gleason grading of prostatic carcinoma. Am J Sur Pathol 2005; 29: 1228–1242.
Cooper EH, Armitage TG, Robinson MR, Newling DW, Richards BR, Smith PH et al. Prostatic specific antigen and the prediction of prognosis in metastatic prostatic cancer. Cancer 1990; 66 (5 Suppl): 1025–1028.
Imai K, Tomaru Y, Ohnuki T, Yamanaka H, Sakai H, Kanetake H et al. Significance of a new stratification of alkaline phosphatase and extent of disease in patients with prostate carcinoma with bone metastasis. Cancer 1992; 69: 2983–2989.
Albertsen PC, Hanley JA, Fine J . 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA 2005; 293: 2095–2101.
Cheng L, Koch MO, Juliar BE, Daggy JK, Foster RS, Bihrle R et al. The combined percentage of Gleason patterns 4 and 5 is the best predictor of cancer progression after radical prostatectomy. J Clin Oncol 2005; 23: 2911–2917.
Collette L, de Reijke TM, Schröder FH . Prostate specific antigen: a prognostic marker of survival in good prognosis metastatic prostate cancer? (EORTC 30892). Eur Urol 2003; 44: 182–189.
Furuya Y, Akakura K, Tobe T, Ichikawa T, Igarashi T, Ito H . Prognostic significance of changes in prostate-specific antigen in patients with metastasis prostate cancer after endocrine treatment. Int Urol Nephrol 2001; 32: 659–663.
Kwak C, Jeong SJ, Park MS, Lee E, Lee SE . Prognostic significance of the nadir prostate specific antigen level after hormone therapy for prostate cancer. J Urol 2002; 168: 995–1000.
Benaim EA, Pace CM, Lam PM, Roehrborn CG . Nadir prostate-specific antigen as a predictor of progression to androgen-independent prostate cancer. Urology 2002; 59: 73–78.
Morote J, Trilla E, Esquena S, Abascal JM, Reventos J . Nadir prostate-specific antigen best predicts the progression to androgen-independent prostate cancer. Int J Cancer 2004; 108: 877–881.
Stewart AJ, Scher HI, Chen MH, McLeod DG, Carroll PR, Moul JW et al. Prostate-specific antigen nadir and cancer-specific mortality following hormonal therapy for prostate-specific antigen failure. J Clin Oncol 2005; 23: 6556–6560.
Robinson D, Sandblom G, Johansson R, Garmo H, Aus G, Hedlund PO et al. PSA kinetics provide improved prediction of survival in metastatic hormone-refractory prostate cancer. Urology 2008; 72: 903–907.
Daskivich TJ, Regan MM, Oh WK . Distinct prognostic role of prostate-specific antigen doubling time and velocity at emergence of androgen independence in patients treated with chemotherapy. Urology 2007; 70: 527–531.
Hussain M, Goldman B, Tangen C, Higano CS, Petrylak DP, Wilding G et al. Prostate-specific antigen progression predicts overall survival in patients with metastatic prostate cancer: data from Southwest Oncology Group Trials 9346 (Intergroup Study 0162) and 9916. J Clin Oncol 2009; 27: 2450–2456.
Usami M, Akaza H, Arai Y, Hirano Y, Kagawa S, Kanetake H et al. Bicalutamide 80 mg combined with a luteinizing hormone-releasing hormone agonist (LHRH-A) versus LHRH-A monotherapy in advanced prostate cancer: findings from a phase III randomized, double-blind, multicenter trial in Japanese patients. Prostate Cancer Prostatic Dis 2007; 10: 194–201.
Schellhammer P, Sharifi R, Block N, Soloway M, Venner P, Patterson AL et al. Maximal androgen blockade for patients with metastatic prostate cancer: outcome of a controlled trial of bicalutamide versus flutamide, each in combination with luteinizing hormone-releasing hormone analogue therapy. Casodex Combination Study Group. Urology 1996; 47 (1A Suppl): 54–60.
Akaza H, Yamaguchi A, Matsuda T, Igawa M, Kumon H, Soeda A et al. Superior anti-tumor efficacy of bicalutamide 80 mg in combination with a luteinizing hormone-releasing hormone (LHRH) agonist versus LHRH agonist monotherapy as first-line treatment for advanced prostate cancer: interim results of a randomized study in Japanese patients. Jpn J Clin Oncol 2004; 34: 20–28.
Prostate Cancer Trialists’ Collaborative Group. Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Lancet 2000; 355: 1491–1498.
Eisenberger MA, Blumenstein BA, Crawford ED, Miller G, McLeod DG, Loehrer PJ et al. Bilateral orchiectomy with or without flutamide for metastatic prostate cancer. N Engl J Med 1998; 339: 1036–1042.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Miyamoto, S., Ito, K., Miyakubo, M. et al. Impact of pretreatment factors, biopsy Gleason grade volume indices and post-treatment nadir PSA on overall survival in patients with metastatic prostate cancer treated with step-up hormonal therapy. Prostate Cancer Prostatic Dis 15, 75–86 (2012). https://doi.org/10.1038/pcan.2011.47
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2011.47
Keywords
This article is cited by
-
Prognostic value of alkaline phosphatase in hormone-sensitive prostate cancer: a systematic review and meta-analysis
International Journal of Clinical Oncology (2020)
-
The prognostic factors of effective ketoconazole treatment for metastatic castration-resistant prostate cancer: who can benefit from ketoconazole therapy?
Asian Journal of Andrology (2012)