Abstract
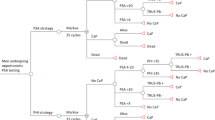
The objective of this study was to evaluate the budget impact of a new prostate cancer risk index for detecting prostate cancer. The index is calculated as the combination of serum prostate-specific antigen (PSA), free PSA and a precursor form p2PSA. We constructed two budget impact models using PSA cutoff values of ⩾2 ng ml−1 (model #1) and ⩾4 ng ml−1 (model #2) for recommending a prostate biopsy in a hypothetical health plan with 100 000 male members aged 50–75 years old. The budgetary impact on the 1-year expected total costs for prostate cancer detection was calculated. Adding the index to the current PSA prostate cancer testing strategies including the total PSA and percent free PSA, the number of detected cancer cases decreased by 20 and 5, in models #1 and #2, respectively. The savings on expected 1-year cost for prostate cancer detection were $356 647 (or $0.30 per-member-per-month (PMPM)) in model #1 and $94 219 ($0.08 PMPM) in model #2. The index produced higher cost savings in the model #1 with PSA cutoff ⩾2 ng ml−1 than the model #2 with cutoff ⩾4 ng ml−1 with a small short-term reduction in the number of positive tests.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ . Cancer statistics, 2009. CA Cancer J Clin 2009; 59: 225–249.
Stark JR, Mucci L, Rothman KJ, Adami HO . Screening for prostate cancer remains controversial. BMJ 2009; 339: b3601.
Greene KL, Albertsen PC, Babaian RJ, Carter HB, Gann PH, Han M et al. Prostate specific antigen best practice statement: 2009 update. J Urol 2009; 182: 2232–2241.
Wolf AM, Wender RC, Etzioni RB, Thompson IM, D'Amico AV, Volk RJ et al. American Cancer Society guidelines for the early detection of cancer: update 2010. Ca Cancer J Clin 2010; 60: 70–98.
Bretton PR . Prostate-specific antigen and digital rectal examination in screening for prostate cancer—a community-based study. Southern Med J 1994; 87: 720–723.
Muschenheim F, Omarbasha B, Kardjian PM, Mondou EN . Screening for carcinoma of the prostate with prostate specific antigen. Ann Clin Lab Sci 1991; 21: 371–380.
Richie JP, Catalona WJ, Ahmann FR, Hudson MA, Scardino PT, Flanigan RC et al. Effect of patient age on early detection of prostate cancer with serum prostate-specific antigen and digital rectal examination. Urology 1993; 42: 365–374.
Brawer MK . Prostate-specific antigen: current status. Ca-Cancer J Clin 1999; 49: 264–281.
Thompson IM, Ankerst DP, Chi C, Lucia MS, Goodman PJ, Crowley JJ et al. Operating characteristics of prostate-specific antigen in men with an initial PSA level of 3.0 ng/ml or lower. JAMA 2005; 294: 66–70.
Nadler RB, Loeb S, Roehl KA, Antenor JA, Eggener S, Catalona WJ . Use of 2.6 Ng/Ml prostate specific antigen prompt for biopsy in men older than 60 years. J Urol 2005; 174: 2154–2157.
Makarov DV, Humphreys EB, Mangold LA, Walsh PC, Partin AW, Epstein JI et al. Pathological outcomes and biochemical progression in men with T1c prostate cancer undergoing radical prostatectomy with prostate specific antigen 2.6 to 4.0 vs 4.1 to 6.0 ng/ml. J Urol 2006; 176: 554–558.
Lucia MS, Darke AK, Goodman PJ, La Rosa FG, Parnes HL, Ford LG et al. Pathologic characteristics of cancers detected in The Prostate Cancer Prevention Trial: implications for prostate cancer detection and chemoprevention. Cancer Prev Res (Phila Pa) 2008; 1: 167–173.
Thompson IM, Ankerst DP . Prostate-specific antigen in the early detection of prostate cancer. CMAJ 2007; 176: 1853–1858.
Brawley OW, Ankerst DP, Thompson IM . Screening for prostate cancer. CA Cancer J Clin 2009; 59: 264–273.
Makarov DV, Loeb S, Getzenberg RH, Partin AW . Biomarkers for prostate cancer. Annu Rev Med 2009; 60: 139–151.
Bangma CH, van Schaik RH, Blijenberg BG, Roobol MJ, Lilja H, Stenman UH . On the use of prostate-specific antigen for screening of prostate cancer in European Randomised Study for Screening of Prostate Cancer. Eur J Cancer 2010; 46: 3109–3119.
Vickers A, Cronin A, Roobol M, Savage C, Peltola M, Pettersson K et al. Reducing unnecessary biopsy during prostate cancer screening using a four-kallikrein panel: an independent replication. J Clin Oncol 2010; 28: 2493–2498.
Sokoll LJ, Chan DW, Mikolajczyk SD, Rittenhouse HG, Evans CL, Linton HJ et al. Proenzyme PSA for the early detection of prostate cancer in the 2.5–4.0 ng/ml total PSA range: preliminary analysis. Urology 2003; 61: 274–276.
Khan MA, Partin AW, Rittenhouse HG, Mikolajczyk SD, Sokoll LJ, Chan DW et al. Evaluation of proprostate specific antigen for early detection of prostate cancer in men with a total prostate specific antigen range of 4.0 to 10.0 ng/ml. J Urol 2003; 170: 723–726.
Sokoll LJ, Wang Y, Feng Z, Kagan J, Partin AW, Sanda MG et al. 2]proenzyme prostate specific antigen for prostate cancer detection: a national cancer institute early detection research network validation study. J Urol 2008; 180: 539–543.
Catalona WJ, Partin AW, Sanda MG, Wei JT, Klee GG, Bangma CH et al. A multicenter study of [-2]Pro-prostate-specific antigen combined with prostate specific antigen and free prostate specific antigen for prostate cancer detection in the 2.0 to 10.0 ng/ml prostate specific antigen range. J Urol 2011; 185: 1650–1655.
Jansen FH, van Schaik RH, Kurstjens J, Horninger W, Klocker H, Bektic J et al. Prostate-specific antigen (PSA) isoform p2PSA in combination with total PSA and free PSA improves diagnostic accuracy in prostate cancer detection. Eur Urol 2010; 57: 921–927.
Le BV, Griffin CR, Loeb S, Carvalhal GF, Kan D, Baumann NA et al. [-2]Proenzyme prostate specific antigen is more accurate than total and free prostate specific antigen in differentiating prostate cancer from benign disease in a prospective prostate cancer screening study. J Urol 2010; 183: 1355–1359.
Ross LE, Coates RJ, Breen N, Uhler RJ, Potosky AL, Blackman D et al. Prostate-specific antigen test use reported in the 2000 National Health Interview Survey. Prev Med 2004; 38: 732–744.
Ross LE, Berkowitz Z, Ekwueme DU . Use of the prostate-specific antigen test among US men: findings from the 2005 national health interview survey. Cancer Epidem Biomar 2008; 17: 636–644.
Lacher DA, Thompson TD, Hughes JP, Saraiya M . Total, free, and percent free prostate-specific antigen levels among U.S. men, 2001–04. Adv Data 2006; 379: 1–12.
Crawford ED, DeAntoni EP, Etzioni R, Schaefer VC, Olson RM, Ross CA . Serum prostate-specific antigen and digital rectal examination for early detection of prostate cancer in a national community-based program. The Prostate Cancer Education Council. Urology 1996; 47: 863–869.
Crawford ED, Abrahamsson PA . PSA-based screening for prostate cancer: how does it compare with other cancer screening tests? Eur Urol 2008; 54: 262–273.
Catalona WJ, Bartsch G, Rittenhouse HG, Evans CL, Linton HJ, Horninger W et al. Serum pro-prostate specific antigen preferentially detects aggressive prostate cancers in men with 2 to 4 ng/ml prostate specific antigen. J Urol 2004; 171 (Pt 1): 2239–2244.
Catalona WJ, Loeb S . Prostate cancer screening and determining the appropriate prostate-specific antigen cutoff values. J Natl Compr Canc Netw 2010; 8: 265–270.
Catalona WJ, Smith DS, Ratliff TL, Dodds KM, Coplen DE, Yuan JJ et al. Measurement of prostate-specific antigen in serum as a screening-test for prostate-cancer. N Engl J Med 1991; 324: 1156–1161.
Thompson IM, Ankerst DP, Chi C, Goodman PJ, Tangen CM, Lucia MS et al. Assessing prostate cancer risk: results from the Prostate Cancer Prevention Trial. J Natl Cancer Inst 2006; 98: 529–534.
Andriole GL, Levin DL, Crawford ED, Gelmann EP, Pinsky PF, Chia D et al. Prostate cancer screening in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial: findings from the initial screening round of a randomized trial. J Natl Cancer Inst 2005; 97: 433–438.
Smith DS, Bullock AD, Catalona WJ . Racial differences in operating characteristics of prostate cancer screening tests. J Urol 1997; 158: 1861–1865.
Mauskopf JA, Sullivan SD, Annemans L, Caro J, Mullins CD, Nuijten M et al. Principles of good practice for budget impact analysis: report of the ISPOR Task Force on good research practices—budget impact analysis. Value Health 2007; 10: 336–347.
Wolters T, van der Kwast TH, Vissers CJ, Bangma CH, Roobol M, Schröder FH et al. False-negative prostate needle biopsies: frequency, histopathologic features, and follow-up. Am J Surg Pathol 2010; 34: 35–43.
Hugosson J, Aus G, Lilja H, Lodding P, Pihl CG, Pileblad E . Prostate specific antigen based biennial screening is sufficient to detect almost all prostate cancers while still curable. J Urol 2003; 169: 1720.
van der Cruijsen-Koeter IW, van der Kwast TH, Schroder FH . Interval carcinomas in the European Randomized Study of Screening for Prostate Cancer (ERSPC)-Rotterdam. J Natl Cancer Inst 95; 1462: 2003.
Hoedemaeker RF, van der Kwast TH, Boer R, de Koning HJ, Roobol M, Vis AN et al. Pathologic features of prostate cancer found at population-based screening with a four-year interval. J Natl Cancer Inst 2001; 93: 1153.
Postma R, Roobol M, Schroder FH, van der Kwast TH . Potentially advanced malignancies detected by screening for prostate carcinoma after an interval of 4 years. Cancer 2004; 100: 968.
Hugosson J, Aus G, Lilja H, Lodding P, Pihl CG . Results of a randomized, population-based study of biennial screening using serum prostate-specific antigen measurement to detect prostate carcinoma. Cancer 2004; 100: 1397.
Schröder FH, Bangma CH, Roobol MJ . Is it necessary to detect all prostate cancers in men with serum PSA levels <3.0 ng/ml? A comparison of biopsy results of PCPT and outcome-related information from ERSPC. Eur Urol 2008; 53: 901–908.
Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL et al. Prevalence of prostate cancer among men with a prostate-specific antigen level <=4.0 ng per milliliter. N Engl J Med 2004; 350: 2239–2246.
Krumholtz JS, Carvalhal GF, Ramos CG, Smith DS, Thorson P, Yan Y et al. Prostate-specific antigen cutoff of 2.6 ng/ml for prostate cancer screening is associated with favorable pathologic tumor features. Urology 2002; 60: 469–473 discussion 473–4.
Meeks JJ, Loeb S, Helfand BT, Kan D, Smith ND, Catalona WJ . Characteristics of prostate cancers detected at prostate specific antigen levels less than 2.5 ng/ml. J Urol 2009; 181: 2515–2518.
Kaplan DJ, Boorjian SA, Ruth K, Egleston BL, Chen DY, Viterbo R et al. Evaluation of the prostate cancer prevention trial risk calculator in a high-risk screening population. Bju Int 2010; 105: 334–337.
Acknowledgements
Partial contents of this research have been presented at the International Society for Pharmacoeconomics and Outcomes Research (ISPOR) 15th Annual International Meeting, Atlanta, GA, May 2010. This study was funded by Beckman Coulter.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Research funding for this study was provided by Beckman Coulter. Dr Michael B Nichol has received research grant from Beckman; Joanne Wu and Jae Jin An have been paid from the research funding from Beckman Coulter in connection with this paper; Joice Huang and Dwight Denham are both employees of Beckman Coulter.
Rights and permissions
About this article
Cite this article
Nichol, M., Wu, J., An, J. et al. Budget impact analysis of a new prostate cancer risk index for prostate cancer detection. Prostate Cancer Prostatic Dis 14, 253–261 (2011). https://doi.org/10.1038/pcan.2011.16
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2011.16
Keywords
This article is cited by
-
Impact of PSA testing on secondary care costs in England and Wales: estimates from the Cluster randomised triAl of PSA testing for Prostate cancer (CAP)
BMC Health Services Research (2023)
-
Cost analysis of prostate cancer detection including the prostate health index (phi)
World Journal of Urology (2019)
-
Budget Impact Analysis of Cancer Screening: A Methodological Review
Applied Health Economics and Health Policy (2019)
-
Clinical utility of the Prostate Health Index (phi) for biopsy decision management in a large group urology practice setting
Prostate Cancer and Prostatic Diseases (2018)
-
Prostate-specific antigen (PSA) isoform p2PSA in prostate cancer screening: systematic review of current evidence and further perspectives
La Rivista Italiana della Medicina di Laboratorio - Italian Journal of Laboratory Medicine (2012)