Abstract
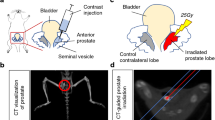
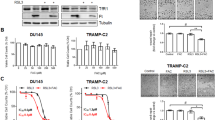
Cryotherapy has emerged as a primary treatment option for prostate cancer (CaP); however, incomplete ablation in the periphery of the cryogenic lesion can lead to recurrence. Accordingly, we investigated the use of a non-toxic adjunctive agent, vitamin D3 (VD3), with cryotherapy to sensitize CaP to low temperature-induced, non-ice rupture-related cell death. VD3 (calcitriol) has been identified as a possible adjunct in the treatment of cancer because of its antiproliferative and antitumorigenic properties. This study aimed to identify the cellular responses and molecular pathways activated when VD3 (calcitriol) is combined with cryotherapy in a murine CaP model. Single freeze–thaw events above −15 °C had little effect on cancer cell viability; however, pretreatment with calcitriol in conjunction with cryo significantly increased cell death. The −15 °C calcitriol combination increased cell death to 55% following a single freeze compared with negligible cell loss by freezing or calcitriol alone. Repeated cryo combination yielded 90% cell death compared with 65% in dual freeze-only cycles. Western blot analysis following calcitriol cryosensitization regimes confirmed the activation of apoptosis. Specifically, proapoptotic Bid and procaspase-3 were found to decrease at 1 h following combination treatment, indicating cleavage to the active forms. A parallel in vivo study confirmed the increased cell death when combining cryotherapy with calcitriol pretreatment. The development of an adjunctive therapy combining calcitriol and cryotherapy represents a potentially highly effective, less toxic, minimally invasive treatment option. These results suggest a role for calcitriol and cryo as a combinatorial treatment for CaP, with the potential for clinical translation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
$259.00 per year
only $64.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Horner MJ, Ries LG, Krapcho M, Neyman N, Aminou R, Howlader N et al. SEER Cancer Statistics Review 1975–2006 National Cancer Institute: Bethesda, MD.
Babaian RJ, Donnelly B, Bahn D, Baust JG, Dineen M, Ellis D et al. Best practice statement on cryosurgery for the treatment of localized prostate cancer. J Urol 2008; 180: 1993–2004.
Cohen JK, Miller RJJ, Ahmed S, Lotz MJ, Baust J . Ten-year biochemical disease control for patients with prostate cancer treated with cryosurgery as primary therapy. Urology 2008; 71: 515–518.
Saliken JC, Donnelly BJ, Rewcastle JC . The evolution and state of modern technology for prostate cryosurgery. Urology 2002; 60 (2 Suppl 1): 26–33.
Gage AA, Baust JG . Cryosurgery for tumors—a clinical overview. Technol Cancer Res Treat 2004; 3: 187–199.
Clarke DM, Baust JM, Van Buskirk RG, Baust JG . Addition of anticancer agents enhances freezing-induced prostate cancer cell death: implications of mitochondrial involvement. Cryobiology 2004; 49: 45–61.
Clarke DM, Baust JM, Van Buskirk RG, Baust JG . Chemo-cryo combination therapy: an adjunctive model for the treatment of prostate cancer. Cryobiology 2001; 42: 274–285.
Clarke DM, Hollister WR, Baust JG, Van Buskirk RG . Cryosurgical modeling: sequence of freezing and cytotoxic agent application affects cell death. Mol Urol 1999; 3: 25–31.
Baust JG, Gage AA, Clarke D, Baust JM, Van Buskirk R . Cryosurgery—a putative approach to molecular-based optimization. Cryobiology 2004; 48: 190–204.
Kimura M, Rabbani Z, Mouraviev V, Tsivian M, Caso J, Satoh T et al. Role of Vitamin D(3) as a sensitizer to cryoablation in a murine prostate cancer model: preliminary in vivo study. Urology 2010; 76: 764, e14-20.
Baust JM, Klossner DP, Robilotto AT, Van Buskirk RG, Gage AA, Mouraviev V et al. Vitamin D3 therapy increases cryoablation efficacy: a novel strategy for the treatment of prostate cancer. British Journal of Urology 2010 (in press).
McCullough ML, Bostick RM, Mayo TL . Vitamin D gene pathway polymorphisms and risk of colorectal, breast, and prostate cancer. Annu Rev Nutr 2009; 29: 111–132.
Norman AW . From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr 2008; 88: 491S–499S.
Peehl DM, Shinghal R, Nonn L, Seto E, Krishnan AV, Brooks JD et al. Molecular activity of 1,25-dihydroxyvitamin D3 in primary cultures of human prostatic epithelial cells revealed by cDNA microarray analysis. J Steroid Biochem Mol Biol 2004; 92: 131–141.
Feldman D, Malloy PJ, Krishnan AV, Balint E . Vitamin D: biology, action and clinical implications. In: Marcus R, Feldman D, Nelson DA, Rosen CJ (eds). Osteoporosis. Acedemic Press: San Diego, 2007, pp 317–382.
Osborne JE, Hutchinson PE . Vitamin D and systemic cancer: is this relevant to malignant melanoma? Br J Dermatol 2002; 147: 197–213.
Krishnan AV, Feldman D . Molecular pathways mediating the anti-inflammatory effects of calcitriol: implications for prostate cancer chemoprevention and treatment. Endocr Relat Cancer 2010; 17: R19–R38.
Montironi R, Thompson D, Scarpelli M, Mazzucchelli R, Peketi P, Hamilton PW et al. Karyometry detects subvisual differences in chromatin organization state between cribriform and flat high-grade prostatic intraepithelial neoplasia. Mod Pathol 2004; 17: 928–937.
Schwartz GG, Whitlatch LW, Chen TC, Lokeshwar BL, Holick MF . Human prostate cells synthesize 1,25-dihydroxyvitamin D3 from 25-hydroxyvitamin D3. Cancer Epidemiol Biomarkers Prev 1998; 7: 391–395.
Hsu JY, Feldman D, McNeal JE, Peehl DM . Reduced 1alpha-hydroxylase activity in human prostate cancer cells correlates with decreased susceptibility to 25-hydroxyvitamin D3-induced growth inhibition. Cancer Res 2001; 61: 2852–2856.
Johnson CS, Hershberger PA, Trump DL . Vitamin D-related therapies in prostate cancer. Cancer Metastasis Rev 2002; 21: 147–158.
Park WH, Seol JG, Kim ES, Hyun JM, Jung CW, Lee CC et al. Induction of apoptosis by vitamin D3 analogue EB1089 in NCI-H929 myeloma cells via activation of caspase 3 and p38 MAP kinase. Br J Haematol 2000; 109: 576–583.
Welsh J . Vitamin D3 and its receptor in mammary gland: from normal development to breast cancer. In: Norman AW, Bouillon R, Thomasset M (eds). Vitamion D Endocrine System. Structural, Biological, Genetic and Clinical Aspects. University of Califronia: Riverside, CA, 2000), pp 453–460.
Narvaez CJ, Welsh J . Role of mitochondria and caspases in vitamin D-mediated apoptosis of MCF-7 breast cancer cells. J Biol Chem 2001; 276: 9101–9107.
Thompson TC, Timme TL, Park SH, Yang G, Ren C . Mouse prostate reconstitution model system: a series of in vivo and in vitro models for benign and malignant prostatic disease. Prostate 2000; 43: 248–254.
Baley PA, Yoshida K, Qian W, Sehgal I, Thompson TC . Progression to androgen insensitivity in a novel in vitro mouse model for prostate cancer. J Steroid Biochem Mol Biol 1995; 52: 403–413.
Klossner DP, Baust JM, Van Buskirk RG, Gage AA, Baust JG . Cryoablative response of prostate cancer cells is influenced by androgen receptor expression. BJU Int 2008; 101: 1310–1316.
Thakur MK, Paramanik V . Role of steroid hormone coregulators in health and disease. Horm Res 2009; 71: 194–200.
Beer TM . Development of weekly high-dose calcitriol based therapy for prostate cancer. Urol Oncol 2003; 21: 399–405.
Lammers T, Peschke P, Kuhnlein R, Subr V, Ulbrich K, Huber P et al. Effect of intratumoral injection on the biodistribution and the therapeutic potential of HPMA copolymer-based drug delivery systems. Neoplasia 2006; 8: 788–795.
Lee J, Youn JI . The photoprotective effect of 1,25-dihydroxyvitamin D3 on ultraviolet light B-induced damage in keratinocyte and its mechanism of action. J Dermatol Sci 1998; 18: 11–18.
Stambolsky P, Tabach Y, Fontemaggi G, Weisz L, Maor-Aloni R, Siegfried Z et al. Modulation of the vitamin D3 response by cancer-associated mutant p53. Cancer Cell 2010; 17: 273–285.
Acknowledgements
The authors would like to acknowledge that this work has been partially funded by the CPSI Biotech and the National Institutes of Health, grant numbers R43CA1123993-01A1 and R43CA118537-01A1.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
In the spirit of full disclosure, the authors declare that Dr KL Santucci receives compensation as a consultant for CPSI Biotech; Dr KK Snyder, Dr JM Baust and Dr RG Van Buskirk are employed by CPSI Biotech; Dr JG Baust, Dr V Mouraviev, Dr TJ Polascik and Dr AA Gage declare no conflict of interest.
Additional information
This work is an original research article and has not been previously published or submitted for publication elsewhere.
Rights and permissions
About this article
Cite this article
Santucci, K., Snyder, K., Baust, J. et al. Use of 1,25α dihydroxyvitamin D3 as a cryosensitizing agent in a murine prostate cancer model. Prostate Cancer Prostatic Dis 14, 97–104 (2011). https://doi.org/10.1038/pcan.2010.52
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/pcan.2010.52
Keywords
This article is cited by
-
Re-purposing cryoablation: a combinatorial ‘therapy’ for the destruction of tissue
Prostate Cancer and Prostatic Diseases (2015)