Abstract
The tumor microenvironment has a dynamic and usually cancer-promoting function during all tumorigenic steps. Glioblastoma (GBM) is a fatal tumor of the central nervous system, in which a substantial number of non-tumoral infiltrated cells can be found. Astrocytes neighboring these tumor cells have a particular reactive phenotype and can enhance GBM malignancy by inducing aberrant cell proliferation and invasion. The tumor suppressor p53 has a potential non-cell autonomous function by modulating the expression of secreted proteins that influence neighbor cells. In this work, we investigated the role of p53 on the crosstalk between GBM cells and astrocytes. We show that extracellular matrix (ECM) from p53+/− astrocytes is richer in laminin and fibronectin, compared with ECM from p53+/+ astrocytes. In addition, ECM from p53+/− astrocytes increases the survival and the expression of mesenchymal markers in GBM cells, which suggests haploinsufficient phenotype of the p53+/– microenvironment. Importantly, conditioned medium from GBM cells blocks the expression of p53 in p53+/+ astrocytes, even when DNA was damaged. These results suggest that GBM cells create a dysfunctional microenvironment based on the impairment of p53 expression that in turns exacerbates tumor endurance.
Similar content being viewed by others
Introduction
The influence of the microenvironment on tumor cells’ behavior is a subject that has been recognized as an important factor modulating tumor malignancy. Nowadays, it is well accepted that non-malignant cells surrounding tumor masses have an important, and often tumor-promoting role during all the stages of carcinogenesis.1 In epithelial tumors, cancer-associated fibroblasts are known to secret soluble factors, such as hepatocyte growth factor, which is mitogenic for malignant cells,2 and also, transforming growth factor beta, which induces epithelial-to-mesenchymal transition and invasion of tumor cells.3 In this same context, tumor-associated macrophages often exhibit a tumor-promoting cytokine expression profile, such as a high expression of IL-10 and a low expression of IL-12.4 Besides secretion of soluble factors, the extracellular matrix (ECM) provides, not only a physical scaffold for all cells in the tumor microenvironment but also has a dynamic role during the evolution and spread of cancers.5 Cancer-associated fibroblasts are known to produce ECM components such as type I and III collagens and fibronectin, which are correlated with a poor prognosis and enhanced metastatic potential.6
Glioblastomas (GBM) are malignant tumors of the central nervous system, with a very inexpressive response to current therapeutic approaches.7 GBM cells are radio- and chemo-resistant; therefore, ways to increase cell death are one of the major targets in GBM research.8, 9, 10 Moreover, the parenchymal infiltration of GBM cells makes total surgical resection an impossible task,11 rendering the study of tumor invasion mechanisms an issue regarding GBM therapy, besides the study of survival mechanisms of GBM cells.7
In surgically resected GBM tissue, a considerable mass of non-transformed cells can be found together with the tumor cells,12 revealing the expressiveness of tumor cells interaction with non-tumoral cells. Astrocytes surrounding GBM commonly present a particular reactive phenotype with high expression of the glial fibrilary acidic protein and have been shown to facilitate the migration of GBM cells by the expression of metalloproteinase 2.13 It has also been suggested that astrocytes may induce aberrant proliferation of GBM cells by secretion of the stromal cell-derived factor 1.12 Thus, the study of GBM and parenchymal astrocytes interaction is relevant concerning tumor therapy.
p53 is a well-known tumor suppressor gene, found mutated in almost 50% of all human cancers, and in 87% of GBM cases.14 In normal conditions, p53 protein has a very-short half-life.15 The role of p53 in DNA damage-induced responses is well studied and established.16,17 Briefly, healthy cells in the presence of DNA damage, have their p53 stabilized, mostly by the disruption of its interaction with the ubiquitin ligase MDM2, post-translationally modified, and accumulated in the nucleus, where it activates the transcription of genes that lead to growth arrest or cell death.18,19 DNA damage-induced apoptosis is completely abolished in the p53−/−central nervous system during development, and is significantly reduced in p53 heterozygous mutant, showing that apoptotic response exhibits p53-haploid insufficiency.20
Interestingly, recent evidences are pointing out that, besides its cell-autonomous function, p53 also exerts a non-cell autonomous function, by the regulation of secreted proteins that can influence the behavior of neighboring cells.21,22 Consistently, Kiaris et al.23 have shown that xenografted epithelial tumor cells exhibited a lower apoptotic rate when injected in a p53−/− host. Inactivation of p53 also occurs within the tumor microenvironment, including cancer-associated fibroblasts, that are associated with an increased rate of metastases and poor prognosis.24,25 Thus, stromal p53 may play an important role in the crosstalk between tumor and surrounding non-tumoral cells.
In this work, we investigated the role of astrocytic p53-expression for GBM malignancy.
Results and Discussion
p53+/− astrocytic extracellular matrix presents more laminin and fibronectin than the one from p53+/+
The importance of microenvironment for the growth of tumor has been observed during tumorigenesis. For example, in fibroblasts, p53 is able to modulate the composition of their own ECM.26 Thus, within the GBM microenvironment, we asked whether, in astrocytes, p53 was able to modulate the composition of their own ECM, which could imply some advantage for GBM cells. To answer these questions, we used astrocytes from mice heterozygous for p53 gene (Trp53) p53+/− 27 and control ones (p53+/+), as a way to analyze the role of reduced or full p53 expression in astrocytes for GBM.
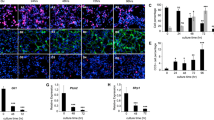
First, we compared extracellular matrices produced by cerebral cortex astrocytes from newborn p53+/+ and p53+/− littermate mice. Our results showed, by immunofluorescence (Figure 1a) and western blot (Figure 1b) that the expression of the ECM components, fibronectin and laminin, were increased in p53+/− astrocytes, compared with p53+/+ astrocytes, a phenotype similar to the observed in reactive astrocytes.28,29
p53+/− astrocytic ECM presents more laminin and fibronectin than p53+/+ astrocytic ECM (a) Representative images of three independent experiments showing immunofluorescence staining of laminin with anti-laminin 1+2 antibody (abcam, Cambridge, UK), (upper panel-green) or fibronectin with anti-fibronectin antibody (Sigma, St Louis, MO, USA) (lower panel-green) in p53+/+ or p53+/− astrocyte ECM. All cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI—blue). Calibration bar: 50 μm. (b) Representative images of three independent western blots showing laminin (Lam) and fibronectin (Fib) expression from p53+/+ and p53+/− astrocytes. α-Tubulin (α-Tub) was used as loading control by anti- α-Tubulin (Sigma antibody at 1:10000 dilution). (c) Histogram showing the levels of astrocytic laminin (black bars) and fibronectin (white bars) proteins. Data represent the mean and error bars of three independent experiments. **P<0.01 and ***P<0.005 by Tukey test.
p53+/− astrocytic ECM promotes GBM cells survival
Since ECM components are able to modulate the malignancy of tumor cells,30 we tested whether GBM cells would have a growth advantage when cultured on ECM produced by p53+/− astrocytes, compared with GBM cells cultured on ECM produced by astrocytes from wild-type littermates. Freshly immobilized ECMs from astrocytes were obtained as previously described.31 Briefly, astrocytes in confluent monolayers were disrupted with cold lysis buffer (PBS-Ca2+, pH 7.4, containing 0.1% Triton X-100, 0.1 M NH4OH, 40 μM leupeptine and 1 mM PMSF) and cellular debris was washed twice with cold PBS-Ca2+.
GBM cell lines T98G (p53 mutant) and U87MG (p53 wild-type) were cultured on ECM produced by p53+/− astrocytes for 24 h. GBM cells cultured on ECM from p53+/− astrocytes showed reduced apoptotic rate when compared with GBM cells cultured on ECM produced by p53+/+ astrocytes. However, no difference was observed in the proliferation of GBM cells in these two culture conditions (Figure 2). Our results show that ECM from p53+/− astrocytes favors GBM cells survival by reducing apoptosis.
p53+/− astrocytic ECM promotes an increase of GBM cell survival compared with p53+/+ astrocytic ECM. (a) Representative photomicrographs of three independent experiments of TUNEL (Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling) assay (red, upper panels) and immunofluorescence staining of Ki67 (green, lower panels) of U87MG cells cultured on p53+/+ or p53+/− astrocytic ECM for 24 h. Freshly immobilized ECMs from astrocytes were obtained as previously described.31 TUNEL assay was performed as described by Borges and co-workers.47 Ki-67 antibody used was purchased from BD Pharmingen, San Diego, CA, USA. All cell nuclei were stained with 4′,6-Diamidino-2-phenylindole (DAPI, blue). TUNEL and ki-67 quantification was done using the percentage of TUNEL or ki-67-positive cells, respectively, relative to total cells (DAPI). Experiments were carried out in duplicates and for every experimental condition at least 500 cells were counted. Cell counting was done by using the Embryonic Stem Cell Counter—ESCC software.48 Arrows indicate TUNEL-positive cells, and arrowheads indicate Ki67-positive cells. Calibration bar: 50 μm. (b) Histogram showing the percentage of U87MG (black bars) and T98G (white bars) TUNEL or Ki67-positive cells, when cultured on p53+/+ and p53+/− astrocytic ECM. Data represent the mean and error bars of three independent experiments. **P<0.01 by Tukey test.
p53+/− astrocytic ECM increases mesenchymal markers in GBM cells
Epithelial-to-mesenchymal transition is a process by which tumor cells acquire a mesenchymal and migratory phenotype.32 This phenomenon can be triggered by growth factors and ECM components like fibronectin and laminin.32,33 Therefore, we investigated whether p53+/− astrocytes ECM could favor the mesenchymal phenotype of GBM cells, besides favoring their survival. To test this, we compared the levels of epithelial (E-cadherin and sprouty 2) and mesenchymal (vimentin and N-cadherin) markers in GBM cells cultured on the ECM from p53+/+ or p53+/− astrocytes. Our results confirmed that, on ECM from p53+/− astrocytes, GBM cells express higher levels of mesenchymal associated proteins than on ECM from p53+/+ astrocytes (Figure 3).
p53+/− astrocytic ECM favors a mesenchymal phenotype of GBM cells. (a) Representative images of three independent western blots showing levels of mesenchymal (N-Cadherin and Vimentin) and epithelial (E-Cadherin and Sprouty-2) phenotype markers of U87MG cells cultured on p53+/+ or p53+/− astrocytic ECM for 24 h. Cyclophilin B was used as loading control. Antibodies used were: anti-N-cadherin (1:1000—BD Pharmingen), anti-E-cadherin (1:1000—BD Pharmingen), anti- Sproty 2 (1:1000—abcam) and anti-vimentin (1:1000—DAKO, Glostrup, Denmark). (b) Representative images of three independent western blots showing levels of mesenchymal (N-Cadherin and Vimentin) and epithelial (Sprouty-2) phenotype markers of T98G cells cultured on p53+/+ or p53+/− astrocyte ECMs. T98G E-cadherin expression is not shown because it was not detectable, as already observed by Mikheeva and co-workers.49 Cyclophilin B was used as loading control. (c) Histogram showing levels of U87MG epithelial-to-mesenchymal transition proteins when GBM cells were cultured on p53+/+ or p53+/− astrocyte ECMs. Data represent the mean and error bars of three independent experiments. ***P<0.005 by Tukey test. (d) Histogram showing levels of T98G epithelial-to-mesenchymal transition proteins when GBM cells were cultured on p53+/+ or p53+/− astrocyte ECM. Data represent the mean and error bars of three independent experiments. **P<0.01 and ***P<0.005 by Tukey test.
The mesenchymal phenotype of tumor cells is linked to resistance to apoptosis 34 and could also indicate an increase in migratory and invasive potentials. Indeed, it has already been shown that fibronectin and laminin increase migration and growth of GBM.35,36
To test whether increased levels of mesenchymal markers, induced by p53+/− ECM, were enough to trigger a change in migration or motility, we performed assays of spontaneous migration and motility in GBM cells cultured on ECM produced by either p53+/+ or p53+/− astrocytes. Migration and motility assays were performed as described previously.37 Briefly, for migration, U87MG cells were cultured on a non-adherent plate for 48 h to form GBM spheres, which were then seeded onto p53+/+ or p53+/− astrocytic ECM. The area of spheres at 6 and 24 h showed no differences in migration (data not shown).
The motility assay was performed using a time-lapse video microscopy. U87MG cells were seeded onto p53+/+ or p53+/− ECM in 96-well plates. Images of live cells were acquired on the Operetta High Content Imaging System equipped with Harmony software (PerkinElmer, Waltham, MA, USA) using a × 20 long wide distance objective in a digital phase contrast mode at a temperature of 37 °C and 5% CO2. Cell motility was monitored by time-lapse image sequence for 90 min at intervals of 2 min. Interestingly, the percentage of migrating U87MG cells on p53+/− ECMs (62.3±6.3% s.e.m.) was increased compared with the cells on p53+/+ ECMs (32.5±12.9% s.e.m.) (n=3, P=0.0285, one-tailed paired t test). The speed of migration and the averages of accumulated distance of the migrating population were similar, regardless of source of ECM. In addition, U87MG cells seem to spread faster over p53+/− ECMs, as suggested by their ratio of width to length and by time-lapse images (Supplementary Information). However, these differences were not statistic significant.
For GBM cells T98G (p53 mutant), however, the percentage of migrating cells, as well as, of all other motility parameters tested by Harmony software in time-lapse images were not different regardless of the ECM tested (not shown). As recently described, an increase in mesenchymal markers is not necessarily translate to increase migration or motility.38 It remains to be tested whether an enhancement of migration phenotype would be more easily observed using ECMs from p53−/− or from p53+/−astrocytes in combination with migration-stimulating factors.
GBM inhibits astrocytic p53-expression to improve cancer survival
In 2009, Bar et al.39 showed that conditioned medium from lung cancer cells suppress p53 expression of fibroblasts. To test whether GBM cells influence the p53 expression of astrocytes, conditioned medium from T98G and U87MG cells were collected after 48 h, as described previously.40 Cerebral cortex astrocytes from newborn wild-type mice were then cultured in these serum-free conditioned media (CM) or in fresh serum-free culture medium (control; CTL), in the presence or absence of 1 μM of the DNA-damaging agent etoposide (VP-16) for 24 h. As expected, etoposide increased p53 expression in wild-type astrocytes (p53+/+), whereas GBM CM reduced p53 expression in astrocytes even after DNA damage. A decrease in p53 levels in astrocytes was also observed in control conditions (without etoposide) when astrocytes were incubated with GBM CM (Figure 4). These results indicate that GBM cells are able to modulate p53 in astocytes.
GBM inhibits astrocytic p53-expression favoring GBM survival. (a) Representative images of three independent western blots showing p53 levels of astrocytes from wild-type cerebral cortex of newborn mice cultured in conditioned medium (CM) made from U87MG (p53 wild type) or T98G (p53 mutant) GBMs cell lines for 24 h. Astrocytes were also cultured in control culture medium (CTL) in the presence or absence of VP-16 (1 μM) for 24 h. Cyclophilin B was used as loading control. (b) Histogram showing the levels of astrocytic p53 protein, when cultured in CM from U87MG or in CTL. Data represent the mean and error bars of three independent experiments. **P<0.01 by t-test. (c) Histogram showing the levels of astrocytic p53 protein, when cultured in CM from U87MG or in CTL in the presence of VP-16 (1 μM), for 24 h. Data represent the mean and error bars of three independent experiments. ***P<0.005 by t-test. (d) Histogram showing the levels of astrocytic p53 protein, when cultured in CM from T98G or in CTL. Data represent the mean and error bars of three independent experiments. **P<0.01 by t-test. (e) Histogram showing the levels of astrocytic p53 protein, when cultured in CM from T98G or in CTL in the presence of VP-16 (1 μM), for 24 h. Data represent the mean and error bars of three independent experiments. ***P<0.005 by t-test. (f) Representative photomicrographs of three independent TUNEL assays (red) of U87MG cells cultured over ECM from p53+/+ astrocytes in CTL. For producing ECMs, p53+/+ astrocytes were incubated in CTL or in the presence of CM of U87MG cells by 72 h, when cell lyses were performed. U87MG cells were then incubated by 24 h over the ECM produced with (right image) or without CM of GBM treatment (left image). Experiments were carried out in duplicates and for every experimental condition at least 500 cells were counted. All nuclei were stained with DAPI (blue). Cell counting was done by using the Embryonic Stem Cell Counter—ESCC software.44 Calibration bar: 50 μm. (g) Histogram showing the percentage of U87MG TUNEL-positive cells cultured in CTL medium for 24 h. U87 cells were cultured over p53+/+ astrocytic ECM that were produced in control media or in the presence of U87MG CM. Data represent the mean and error bars of three independent experiments (*P<0.05 by t-test).
We then tested whether ECM produced by astrocytes, under the influence of GBM CM, increases GBM survival. p53+/+ Astrocytes were cultured with control medium or with CM of U87MG cells for 3 days. U87MG cells were then seeded onto ECM produced by these astrocytes that had been incubated previously with control or GBM CM. GBM cells were then allowed to grow in control medium for 24 h. As shown in Figure 4, CM of GBM made p53+/+ astrocytes more permissive to GBM growth. Moreover, ECM produced by astrocytes under the influence of GBM CM reduced the percentage of terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling-positive GBM cells. Therefore, GBM CM was able to make astrocytes to secrete ECM that increases GBM survival.
Using microarray analysis, Katz et al.41 have shown that astrocytes surrounding GBM have a specific expression pattern, which is different from the pattern of astrocytes not surrounding the tumor. Gagliano et al.42 have also shown that the co-culture of astrocytes with GBM cells increases the expression of MMP-2 and a decrease in TIMP-2 (tissue inhibitor of metalloproteinase) in astrocytes. Our work shows that GBM cells are able to modulate p53 expression of astrocytes, confirming that GBM is able to modulate protein expression of surrounding astrocytes as a way to favor malignancy. Moreover, DNA damage is well known to be present in tumor microenvironment because of hypoxia-reoxygenation cycles and is induced by chemotherapy treatment,43 which makes very interesting to note the reduction of astrocytic p53 expression even after DNA damage.
Recently, it was shown that the loss of p53 in surrounding fibroblasts increases epithelial tumor growth.44, 45, 46 The authors suggest that this phenomenon is dependent on the expression of growth factors44,45 or by the shift of the tumor-associated macrophage phenotype from M1 (classically activated) to M2 (alternatively activated).46 Our work is the first to show that the loss of p53 expression in astrocytes is able to modulate ECM composition and to provide advantages for GBM cells, favoring the expression of mesenchymal markers and cell survival. Our results strengthen the concept that, in a tumor microenvironment, p53 acts as a tumor suppressor not only in the tumor cell itself, but also in the parenchymal cell.
Altogether, we have elucidated a very important crosstalk between GBM cells and surrounding astrocytes. We have shown that GBM cells decrease the expression of p53 in astrocytes, which in turn, modulates ECM composition and favors tumor malignancy. Finally, our data point out an important role of p53 in the interaction between GBM cells and astrocytes, a discovery with potential to conceive new approaches to treat GBM.
References
Hanahan D, Coussens LM . Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 2012; 21: 309–322.
Brittan M, Hunt T, Jeffery R, Poulsom R, Forbes SJ, Hodivala-Dilke K et al. Bone marrow derivation of pericryptal myofibroblasts in the mouse and human small intestine and colon. Gut 2002; 50: 752–757.
Erez N, Truitt M, Olson P, Arron ST, Hanahan D . Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-dependent manner. Cancer Cell 2010; 17: 135–147.
Mantovani A, Allavena P, Sica A, Balkwill F . Cancer-related inflammation. Nature 2008; 454: 436–444.
Lu P, Weaver VM, Werb Z . The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol 2012; 196: 395–406.
Cirri P, Chiarugi P . Cancer associated fibroblasts: the dark side of the coin. Am J Cancer Res 2011; 1: 482–497.
Lima FR, Kahn SA, Soletti RC, Biasoli D, Alves T, da Fonseca AC et al. Glioblastoma: therapeutic challenges, what lies ahead. Biochim Biophys Acta 2012; 1826: 338–349.
Biasoli D, Kahn SA, Cornelio TA, Furtado M, Campanati L, Chneiweiss H et al. Retinoblastoma protein regulates the crosstalk between autophagy and apoptosis, and favors glioblastoma resistance to etoposide. Cell Death Dis 2013; 4: e767.
Kahn SA, Biasoli D, Garcia C, Geraldo LH, Pontes B, Sobrinho M et al. Equinatoxin II potentiates temozolomide and etoposide-induced glioblastoma cell death. Curr Top Med Chem 2012; 12: 2082–2093.
Soletti RC, de Faria GP, Vernal J, Terenzi H, Anderluh G, Borges HL et al. Potentiation of anticancer-drug cytotoxicity by sea anemone pore-forming proteins in human glioblastoma cells. Anticancer Drugs 2008; 19: 517–525.
Schiffer D, Cavalla P, Dutto A, Borsotti L . Cell proliferation and invasion in malignant gliomas. Anticancer Res 1997; 17: 61–69.
Charles NA, Holland EC, Gilbertson R, Glass R, Kettenmann H . The brain tumor microenvironment. Glia 2012; 60: 502–514.
Le DM, Besson A, Fogg DK, Choi KS, Waisman DM, Goodyer CG et al. Exploitation of astrocytes by glioma cells to facilitate invasiveness: a mechanism involving matrix metalloproteinase-2 and the urokinase-type plasminogen activator-plasmin cascade. J Neurosci 2003; 23: 4034–4043.
McLendon R, Friedman A, Bigner D, Van Meir EG, Brat DJ, Mastrogianakis GM et al. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008; 455: 1061–1068.
Ashcroft M, Vousden KH . Regulation of p53 stability. Oncogene 1999; 18: 7637–7643.
Green DR, Kroemer G . Cytoplasmic functions of the tumour suppressor p53. Nature 2009; 458: 1127–1130.
Biderman L, Poyurovsky MV, Assia Y, Manley JL, Prives C . MdmX is required for p53 interaction with and full induction of the Mdm2 promoter after cellular stress. Mol Cell Biol 2012; 32: 1214–1225.
Soussi T . p53 alterations in human cancer: more questions than answers. Oncogene 2007; 26: 2145–2156.
Michael D, Oren M . The p53-Mdm2 module and the ubiquitin system. Semin Cancer Biol 2003; 13: 49–58.
Borges HL, Chao C, Xu Y, Linden R, Wang JY . Radiation-induced apoptosis in developing mouse retina exhibits dose-dependent requirement for ATM phosphorylation of p53. Cell Death Differ 2004; 11: 494–502.
Khwaja FW, Svoboda P, Reed M, Pohl J, Pyrzynska B, Van Meir EG . Proteomic identification of the wt-p53-regulated tumor cell secretome. Oncogene 2006; 25: 7650–7661.
Rangel LP, Costa DC, Vieira TC, Silva JL . The aggregation of mutant p53 produces prion-like properties in cancer. Prion 2014; 8: 1.
Kiaris H, Chatzistamou I, Trimis G, Frangou-Plemmenou M, Pafiti-Kondi A, Kalofoutis A . Evidence for nonautonomous effect of p53 tumor suppressor in carcinogenesis. Cancer Res 2005; 65: 1627–1630.
Hill R, Song Y, Cardiff RD, Van Dyke T . Selective evolution of stromal mesenchyme with p53 loss in response to epithelial tumorigenesis. Cell 2005; 123: 1001–1011.
Patocs A, Zhang L, Xu Y, Weber F, Caldes T, Mutter GL et al. Breast-cancer stromal cells with TP53 mutations and nodal metastases. N Engl J Med 2007; 357: 2543–2551.
Alexandrova A, Ivanov A, Chumakov P, Kopnin B, Vasiliev J . Changes in p53 expression in mouse fibroblasts can modify motility and extracellular matrix organization. Oncogene 2000; 19: 5826–5830.
Jacks T, Remington L, Williams BO, Schmitt EM, Halachmi S, Bronson RT et al. Tumor spectrum analysis in p53-mutant mice. Curr Biol 1994; 4: 1–7.
Egan RA, Vijayan VK . Fibronectin immunoreactivity in neural trauma. Brain Res 1991; 568: 330–334.
Liesi P, Kaakkola S, Dahl D, Vaheri A . Laminin is induced in astrocytes of adult brain by injury. EMBO J 1984; 3: 683–686.
Frantz C, Stewart KM, Weaver VM . The extracellular matrix at a glance. J Cell Sci 2010; 123: 4195–4200.
Alves TR, da Fonseca AC, Nunes SS, da Silva AO, Dubois LG, Faria J et al. Tenascin-C in the extracellular matrix promotes the selection of highly proliferative and tubulogenesis-defective endothelial cells. Exp Cell Res 2011; 317: 2073–2085.
Hanahan D, Weinberg RA . Hallmarks of cancer: the next generation. Cell 2011; 144: 646–674.
Zeisberg M, Neilson EG . Biomarkers for epithelial-mesenchymal transitions. J Clin Invest 2009; 119: 1429–1437.
Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG . Cancer drug resistance: an evolving paradigm. Nature Rev Cancer 2013; 13: 714–726.
Berens ME, Rief MD, Loo MA, Giese A . The role of extracellular matrix in human astrocytoma migration and proliferation studied in a microliter scale assay. Clin Exp Metastasis 1994; 12: 405–415.
Serres E, Debarbieux F, Stanchi F, Maggiorella L, Grall D, Turchi L et al. Fibronectin expression in glioblastomas promotes cell cohesion, collective invasion of basement membrane in vitro and orthotopic tumor growth in mice. Oncogene 2013; 33: 3451–3462.
Marins M, Xavier ALR, Viana NB, Fortes FSA, Fróes MM, Menezes JRL . Gap junctions are involved in cell migration in the early postnatal subventricular zone. Dev Neurobiol 2009; 69: 715–730.
Schaeffer D, Somarelli JA, Hanna G, Palmer GM, Garcia-Blanco MA . Cellular migration and invasion uncoupled: increased migration is not an inexorable consequence of epithelial-to-mesenchymal transition. Mol Cell Biol 2014; 34: 3486–3499.
Bar J, Feniger-Barish R, Lukashchuk N, Shaham H, Moskovits N, Goldfinger N et al. Cancer cells suppress p53 in adjacent fibroblasts. Oncogene 2009; 28: 933–936.
Fonseca AC, Romao L, Amaral RF, Assad Kahn S, Lobo D, Martins S et al. Microglial stress inducible protein 1 promotes proliferation and migration in human glioblastoma cells. Neuroscience 2012; 200: 130–141.
Katz AM, Amankulor NM, Pitter K, Helmy K, Squatrito M, Holland EC . Astrocyte-specific expression patterns associated with the PDGF-induced glioma microenvironment. PLoS ONE 2012; 7: e32453.
Gagliano N, Costa F, Cossetti C, Pettinari L, Bassi R, Chiriva-Internati M et al. Glioma-astrocyte interaction modifies the astrocyte phenotype in a co-culture experimental model. Oncol Rep 2009; 22: 1349–1356.
Pires IM, Bencokova Z, Milani M, Folkes LK, Li JL, Stratford MR et al. Effects of acute versus chronic hypoxia on DNA damage responses and genomic instability. Cancer Res 2010; 70: 925–935.
Trachootham D, Chen G, Zhang W, Lu W, Zhang H, Liu J et al. Loss of p53 in stromal fibroblasts promotes epithelial cell invasion through redox-mediated ICAM1 signal. Free Radic Biol Med 2013; 58: 1–13.
Addadi Y, Moskovits N, Granot D, Lozano G, Carmi Y, Apte RN et al. p53 status in stromal fibroblasts modulates tumor growth in an SDF1-dependent manner. Cancer Res 2010; 70: 9650–9658.
Lujambio A, Akkari L, Simon J, Grace D, Tschaharganeh DF, Bolden JE et al. Non-cell-autonomous tumor suppression by p53. Cell 2013; 153: 449–460.
Borges HL, Hunton IC, Wang JY . Reduction of apoptosis in Rb-deficient embryos via Abl knockout. Oncogene 2007; 26: 3868–3877.
Faustino G, Gattass M, Rehen S, Lucena CJP . Automatic Embryonic Stem Cells Detection and Counting Method in Fluorescence Microscopy Images. ISBI, 2009, pp 799–802.
Mikheeva SA, Mikheev AM, Petit A, Beyer R, Oxford RG, Khorasani L et al. TWIST1 promotes invasion through mesenchymal change in human glioblastoma. Mol Cancer 2010; 9: 194.
Acknowledgements
We thank Rosenilde Carvalho de Holanda Afonso, Fabio Jorge Moreira and Andréa Fantinatti for technical assistance. We thank Dr Loraine Campanati (UFRJ) for donation of anti-N -cadherin; anti- E-cadherin and anti- Sprouty 2 antibodies. This work was supported by the National Council for Scientific and Technological Development (CNPq), by the Brazilian Federal Agency for Support and Evaluation of Higher Education (CAPES), by the Carlos Chagas Filho Foundation for Research Support of the State of Rio de Janeiro (FAPERJ) and Oncobiology Program from UFRJ (Ary Frauzino Foudation – FAF/ONCO).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogenesis website .
Rights and permissions
Oncogenesis is an open-access journal published by Nature Publishing Group. This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 3.0 Unported License. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-nd/3.0/
About this article
Cite this article
Biasoli, D., Sobrinho, M., da Fonseca, A. et al. Glioblastoma cells inhibit astrocytic p53-expression favoring cancer malignancy. Oncogenesis 3, e123 (2014). https://doi.org/10.1038/oncsis.2014.36
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/oncsis.2014.36
This article is cited by
-
Cellular Conversations in Glioblastoma Progression, Diagnosis and Treatment
Cellular and Molecular Neurobiology (2023)
-
Glioma: molecular signature and crossroads with tumor microenvironment
Cancer and Metastasis Reviews (2022)
-
Main genetic differences in high-grade gliomas may present different MR imaging and MR spectroscopy correlates
European Radiology (2021)
-
High filamin-C expression predicts enhanced invasiveness and poor outcome in glioblastoma multiforme
British Journal of Cancer (2019)
-
Multidimensional communication in the microenvirons of glioblastoma
Nature Reviews Neurology (2018)