Abstract
First-hits in the multi-hit process of leukemogenesis originate in germline or hematopoietic stem cells (HSCs), yet leukemia-initiating cells (LICs) usually have a lineage-committed phenotype. The molecular mechanisms underlying this compartment shift during leukemia evolution have not been a major focus of investigation and remain poorly understood. Here a mechanism underlying this shift was examined in the context of Runx1 deficiency, a frequent leukemia-initiating event. Lineage-negative cells isolated from the bone marrow of Runx1-haploinsufficient and wild-type control mice were cultured in granulocyte-colony-stimulating factor to force lineage commitment. Runx1-haploinsufficient cells demonstrated significantly greater and persistent exponential cell growth than wild-type controls. Not surprisingly, the Runx1-haploinsufficient cells were differentiation-impaired, by morphology and by flow-cytometric evaluation for granulocyte differentiation markers. Interestingly, however, this impaired differentiation was not because of decreased granulocyte lineage commitment, as RNA and protein upregulation of the master granulocyte lineage-commitment transcription factor Cebpa, and Hoxb4 repression, was similar in wild-type and Runx1-haploinsufficient cells. Instead, RNA and protein expression of Cebpe, a key driver of progressive maturation after lineage commitment, were significantly decreased in Runx1-haploinsufficient cells. Primary acute myeloid leukemia cells with normal cytogenetics and RUNX1 mutation also demonstrated this phenotype of very high CEBPA mRNA expression but paradoxically low expression of CEBPE, a CEBPA target gene. Chromatin-immunoprecipitation analyses suggested a molecular mechanism for this phenotype: in wild-type cells, Runx1 binding was substantially greater at the Cebpe than at the Cebpa enhancer. Furthermore, Runx1 deficiency substantially diminished high-level Runx1 binding at the Cebpe enhancer, but lower-level binding at the Cebpa enhancer was relatively preserved. Thus, Runx1-deficiency permits Cebpa upregulation and the exponential cell growth that accompanies lineage commitment, but by impairing activation of Cebpe, a key proliferation-terminating maturation gene, extends this exponential growth. These mechanisms facilitate germline cell or HSC of origin, yet evolution into LIC with lineage-committed phenotype.
Similar content being viewed by others
Introduction
Known first-hits in the multi-hit process of leukemogenesis are inactivating or dominant-negative mutations in RUNX1.1 These first-hits originate in the germline in familial acute myeloid leukemia (AML, reviewed in the study by Owen et al.2) and in hematopoietic stem cells (HSCs) in acquired AML.3, 4, 5 Germline or stem cell origin of the first hit is necessary as these cells live long enough that there is a feasible possibility of secondary genetic abnormalities (additional hits) that can cooperate for evolution into AML. However, some of these secondary genetic abnormalities occur in daughter cells with restricted lineage potential. This is evident in familial AML, in which neoplastic evolution does not occur in the germline cells of origin, but is also true of acquired AML: self-renewing AML cells, also known as leukemia-initiating cells (LICs) or leukemia stem cells, have a lineage-committed phenotype by numerous surface markers (CD34+38+, CLL-1+, CD71+, CD90−, c-Kit-),6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16 by strikingly high expression of lineage-specifying transcription factors such as CEBPA, PU.1/SPI1 or GATA1,17, 18, 19 by inactivation of polycomb repressor complex 2 components that usually suppress lineage programs,20 and by the observation that highly recurrent secondary genetic abnormalities such as FLT3 mutation are not detected in the HSC compartment.5
Thus, a first-hit is present or originates in HSC, but creates conditions that favor further neoplastic evolution not necessarily in the HSC compartment itself but in lineage-committed progenitors. The molecular mechanisms underlying this compartment shift during leukemia evolution have not been a major focus of investigation and are poorly understood. A better understanding of these mechanisms could provide guidance for novel treatments that exploit the lineage-committed cellular context of LIC to thereby spare uncommitted normal HSC.
Results and Discussion
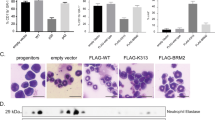
To investigate a basis for compartment shifts during evolution of myeloid cancers, lineage-negative HSC from wild-type and Runx1 haploinsufficient mice21 were cultured in granulocyte-colony-stimulating factor (G-CSF) to force lineage commitment. The Runx1-haploinsufficient cells demonstrated more rapid and persistent proliferation than wild-type controls (Figure 1a). This growth advantage was also apparent in semi-solid media supplemented with G-CSF: Runx1-haploinsufficient cells produced a higher number and larger-sized colonies than wild-type control (Figure 1b). The Runx1-haploinsufficient cells were differentiation-impaired: after 15 days of culture, Runx1-haploinsufficient cells included mitotic figures and immature forms with high nuclear-cytoplasmic ratios and decreased neutrophilic granulation, whereas wild-type cells demonstrated mostly mature granulocytes (Figure 1c). Furthermore, the granulocyte-lineage markers Ly6G and CD11b were upregulated to a much lesser extent in Runx1-haploinsufficient cells than in wild-type controls (Figure 1d).
Runx1-haploinsufficient cells are able to lineage commit but have impaired subsequent maturation. Lineage-negative (Lin−) cells were isolated from bone marrow of Runx1+/− (haploinsufficient) and wild-type (WT) C57BL/6 mice using MACS Lineage Cell Depletion Kit (#130-090-858, Miltenyi Biotec Inc., Auburn, CA, USA). (a) Cell counts. Cytokine supplementation: 10 ng/ml of mSCF and 100 ng/ml of thrombopoietin (non-differentiation promoting conditions) or 10 ng/ml of murine stem cell factor, 10 ng/ml of mIL-6 and G-CSF 20 ng/ml (PeproTech Inc., Rocky Hill, NJ, USA). Cell numbers were determined by automated cell counter every 3 days during 60 days of culture. Mean±standard deviation. Experiments performed in triplicate. (b) Colony formation by Runx1+/− and WT Lin− cells in semi-solid media. 2 × 104cells/ml of methylcellulose (MethoCult GF M3434; Stem Cell Technologies, Vancouver, Canada). Colonies counted and imaged by inverted microscope on day 10 (LeicaDMI6000B inverted microscope, Leica Microsystems, Wetzlar, Germany). Experiments performed in triplicate. ** p<0.01, Wilcoxon test. (c) Runx1+/− and WT cell morphology after 15 days of culture with G-CSF. Cytospins were generated with a Shandon CytoSpinIII (Thermo Scientific, Pittsburgh, PA, USA; 500 r.p.m., 5 min). Air-dryed cells fixed with 100% methanol were Giemsa-stained for visualization using a Leica DMR light microscope equipped with CCD camera (Leica Microsystems), magnification × 630. (d) Ly6G and CD11b granulocyte-lineage marker expression in Runx1+/− and WT Lin− cells on day 0 (D0) and day 7 (D7) of culture with G-CSF. Expression measured by Coulter Epics XL-MCL flow cytometer and CXP software (Beckman-Coulter, Brea, CA, USA). Antibodies used were anti-mouse Ly6G-FITC (eBiosciences, San Diego, CA, USA; #551460), anti-mouse CD11b-PE (eBiosciences, #120112), and isotype-matched immunoglobulin control.
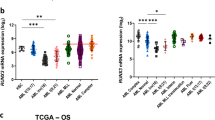
To better understand the molecular mechanisms underlying the persistent proliferation and impaired differentiation, expression patterns of key hematopoietic transcription factors that determine lineage commitment and progressive maturation were examined. These were Hoxb4 that promotes self-renewal by HSC, Cebpa that drives granulocyte lineage commitment, and Cebpe that drives late granulocyte maturation and terminates proliferation.22, 23, 24, 25, 26, 27 Repression of Hoxb4 and activation of Cebpa by G-CSF was similar in wild-type and Runx1-haploinsufficient cells (Figure 2a). Despite the upregulation of Cebpa, Cebpe activation was significantly decreased in the Runx1-haploinsufficient cells, even after 15 days of culture with G-CSF (Figure 2a). These observations were recapitulated at the protein level (Figure 2b). This pattern of gene expression is also a characteristic of primary AML cells with mutated RUNX1 and normal cytogenetics: CEBPA was on average >30-fold more expressed than CEBPE and >6-fold more expressed than HOXB4 (Figure 2c). Chromatin-immunoprecipitation analysis showed a basis for differential Cebpa and Cebpe activation in Runx1-deficient cells: in wild-type cells, Runx1 binding was substantially greater at the Cebpe than at the Cebpa enhancer28, 29 (Figure 2d). Furthermore, Runx1-deficiency substantially diminished high level Runx1 binding at the Cebpe enhancer, but lower level binding at the Cebpa enhancer was relatively preserved (Figure 2d). In other words, RUNX1 is more abundant at, and presumably more important, for regulating the Cebpe enhancer than the Cebpa enhancer.
Unequal impact of Runx1 deficiency on Runx1 binding at the Cebpa and Cebpe enhancers and on Cebpa and Cebpe activation in response to G-CSF. (a) Time-course expression of Hoxb4 (key stem cell transcription factor), Cebpa (key lineage-commitment transcription factor) and Cebpe (key late-differentiation transcription factor) in WT and Runx1+/− lineage-negative (Lin−) cells cultured with G-CSF for 15 days. SYBR Green bound to double-stranded DNA was detected in real-time with the 7500 Fast Real Time PCR System (Applied Biosystems, Foster City, CA, USA). Relative expression values were calculated by raising 2 to the power of the negative value of delta-delta CT for each sample. Primer sequences: HoxB4: forward (F): 5′-CCAGAATCGGCGCATGA-3, reverse (R): 5′-CCCGAGCGGATCTTGGT-3′; Cebpa: F: 5′-CAAAGCCAAGAAGTCGGTGGACAA-3′; R: 5′-TCATTGTCACTGGTCAACTCCAGC-3′; Cebpe: F: 5′-GCTACAATCCCCTGCAGTACC-3′; R: 5′-TGCCTTCTTGCCCTTGTG-3′; GAPDH: F: 5′-ACCACACTCCATGCCATCAC-3′, R:5′-TCCACCACCCTGTTGCTGTA-3′. Mean±standard deviation. **P<0.01, Student’s t-test. Experiments performed in triplicate. (b) Cebpa and Cebpe protein expression in WT and Runx1+/− Lin− cells cultured for 15 days with G-CSF. Western blot analyses with the following primary antibodies: anti-Cebpa, anti-Cebpe (Santa Cruz Biotechnology, Dallas, TX, USA; #SC-61, #SC-25770), anti-β-actin (Sigma-Aldrich, St Louis, MO, USA, A3854); secondary antibodies: anti-Rabbit (GE Healthcare, Waukesha, WI, USA; NA934) and anti-Mouse (GE Healthcare, NXA931) at 1:5000 and 1:10 000 dilutions, respectively. (c) Gene expression of CEBPA, CEBPE and HOXB4 in primary AML cells with normal cytogenetics and RUNX1 mutation (TCGA database, n=14). Heatmap by Arraystar software. (d) Chromatin immunoprecipitation (ChIP) analysis of Runx1+/− and WT cells to evaluate Runx1 binding at the Cepba and Cebpe enhancers. Enhancers of Cebpa and Cebpe were identified by others using ChIP coupled with deep sequencing.28 ChIP performed using ChIP Assay Kit (cat#17-295, EMD Millipore, Billerica, MA, USA). 5 × 106 cells bone marrow (BM) cells from WT and Runx1+/1 fresh BM cells were resuspended in 10 ml of media and formaldehyde was added to a final concentration of 1% followed by 10 min incubation at 37 °C. The media containing formaldehyde was removed and the cells were washed twice with ice-cold PBS containing protease inhibitor (cat#P8340, Sigma-Aldrich) and phosphatase inhibitor cocktails (cat#P0044, Sigma-Aldrich). Crosslinked cells were resuspended in SDS lysis buffer (Millipore, #20–163) and incubated for 10 min on ice. These cell pellets were sonicated at 15 s pulses with 45 s hold on ice for a total of 10 min of pulsing at full power (Fisher-Scientific Model #550 Sonic Dismembrator equipped with a microtip probe). Genomic DNA fragments of 200-bp and 1-kb in size were obtained (confirmed by agarose gel electrophoresis). After centrifugation at 4 °C, the supernatant was pre-cleared with salmon sperm DNA/Protein A agarose-50% slurry (Millipore, #16–157) for 30 min at 4 °C with agitation. A 10% aliquot (vol) of the precleared protein/DNA mixture was removed and used for subsequent reverse transcription–PCR input quantification. 10 μg of anti-Runx1 ChIP grade antibody (cat#ab23980, Abcam, Cambridge, MA, USA) was added to the remaining protein/DNA mixture and incubated at 4 °C overnight with rotation. Salmon sperm DNA/Protein A agarose-50% slurry was added and incubated for 1 h at 4 °C followed by washes with the ChIP assay kit provided buffers. The immunoprecipitated (IP) complexes were reverse crosslinked at 65 °C for 4 h and DNA was recovered by phenol/chloroform extraction protocol (Sigma). The IP products were amplified and quantified using real-time PCR. ChIP primer sequences are:21 cepba enhancer left primer, 5′- TTCCCGTTTCTGAAATCTGC-3′, cebpa enhancer right primer, 5′-GGTTGTGGCAAGAAGGTCAC-3′, cebpe enhancer left primer, 5′-GTGTCATGGTCACCCTAGCC-3′ and cebpe enhancer right primer, 5′-CTGGAGCTAGCAGGGGTTTT-3′. Mean±standard deviation from three independent ChIP experiments. **P<0.001, NS=not significant, Tukey–Kramer HSD test.
Consistent with the present results, Runx1-haploinsufficient mice have decreased HSC but increased myeloid progenitors compared with wild-type controls.21 Similarly, a murine knock-in model of point-mutated Runx1 demonstrated unaltered HSC emergence but defects in multiple committed hematopoietic lineages, and hematopoietic cells containing the leukemia fusion protein RUNX1-ETO demonstrated delayed granulocytic differentiation.30, 31 Previously, we demonstrated that even CD34+38− subsets of primary AML cells demonstrate this pattern of very high CEBPA but relatively low HOXB4 and CEBPE expression compared with normal hematopoietic precursors, promyelocytes and neutrophils.18, 32, 33 Ideally, leukemia treatments would suppress malignant clones but preserve normal HSC needed to restore blood counts. High expression of key transcription factors that drive differentiation (for example, CEBPA, PU.1) could be a difference between LIC and normal HSC that facilitates this goal, especially since the proliferation-terminating differentiation genes usually activated by these transcription factors, although aberrantly suppressed in leukemia cells (for example, CEBPE), are genetically intact and thus in theory available for activation. For example, high baseline CEBPA expression explains rapid restoration of CEBPE expression and cycle exit by maturation of primary AML cells treated with corepressor antagonizing drugs (for example, decitabine) or with FLT3 inhibitors.34 The same treatments preserve self-renewal of uncommitted normal HSC that do not express high levels of lineage-specifying transcription factors at baseline.35, 36, 37
References
Osato M, Asou N, Abdalla E, Hoshino K, Yamasaki H, Okubo T et al. Biallelic and heterozygous point mutations in the runt domain of the AML1/PEBP2alphaB gene associated with myeloblastic leukemias. Blood 1999; 93: 1817–1824.
Owen C, Barnett M, Fitzgibbon J . Familial myelodysplasia and acute myeloid leukaemia--a review. Br J Haematol 2008; 140: 123–132.
Miyamoto T, Weissman IL, Akashi K . AML1/ETO-expressing nonleukemic stem cells in acute myelogenous leukemia with 8;21 chromosomal translocation. Proc Natl Acad Sci USA 2000; 97: 7521–7526.
Wiemels JL, Xiao Z, Buffler PA, Maia AT, Ma X, Dicks BM et al. In utero origin of t(8;21) AML1-ETO translocations in childhood acute myeloid leukemia. Blood 2002; 99: 3801–3805.
Jan M, Snyder TM, Corces-Zimmerman MR, Vyas P, Weissman IL, Quake SR et al. Clonal evolution of preleukemic hematopoietic stem cells precedes human acute myeloid leukemia. Sci Transl Med 2012; 4: 149ra118.
Taussig DC, Miraki-Moud F, Anjos-Afonso F, Pearce DJ, Allen K, Ridler C et al. Anti-CD38 antibody-mediated clearance of human repopulating cells masks the heterogeneity of leukemia-initiating cells. Blood 2008; 112: 568–575.
Kirstetter P, Schuster MB, Bereshchenko O, Moore S, Dvinge H, Kurz E et al. Modeling of C/EBPalpha mutant acute myeloid leukemia reveals a common expression signature of committed myeloid leukemia-initiating cells. Cancer Cell 2008; 13: 299–310.
Huntly BJ, Shigematsu H, Deguchi K, Lee BH, Mizuno S, Duclos N et al. MOZ-TIF2, but not BCR-ABL, confers properties of leukemic stem cells to committed murine hematopoietic progenitors. Cancer Cell 2004; 6: 587–596.
Somervaille TC, Cleary ML . Identification and characterization of leukemia stem cells in murine MLL-AF9 acute myeloid leukemia. Cancer Cell 2006; 10: 257–268.
van Rhenen A, Moshaver B, Kelder A, Feller N, Nieuwint AW, Zweegman S et al. Aberrant marker expression patterns on the CD34+. Leukemia 2007; 21: 1700–1707.
Blair A, Hogge DE, Ailles LE, Lansdorp PM, Sutherland HJ . Lack of expression of Thy-1 (CD90) on acute myeloid leukemia cells with long-term proliferative ability in vitro and in vivo. Blood 1997; 89: 3104–3112.
Blair A, Sutherland HJ . Primitive acute myeloid leukemia cells with long-term proliferative ability in vitro and in vivo lack surface expression of c-kit (CD117). Exp Hematol 2000; 28: 660–671.
Feuring-Buske M, Gerhard B, Cashman J, Humphries RK, Eaves CJ, Hogge DE . Improved engraftment of human acute myeloid leukemia progenitor cells in beta 2-microglobulin-deficient NOD/SCID mice and in NOD/SCID mice transgenic for human growth factors. Leukemia 2003; 17: 760–763.
Wunderlich M, Chou FS, Link KA, Mizukawa B, Perry RL, Carroll M et al. AML xenograft efficiency is significantly improved in NOD/SCID-IL2RG mice constitutively expressing human SCF, GM-CSF and IL-3. Leukemia 2010; 24: 1785–1788.
Agliano A, Martin-Padura I, Mancuso P, Marighetti P, Rabascio C, Pruneri G et al. Human acute leukemia cells injected in NOD/LtSz-scid/IL-2Rgamma null mice generate a faster and more efficient disease compared to other NOD/scid-related strains. Int J Cancer 2008; 123: 2222–2227.
Sarry JE, Murphy K, Perry R, Sanchez PV, Secreto A, Keefer C et al. Human acute myelogenous leukemia stem cells are rare and heterogeneous when assayed in NOD/SCID/IL2Rgammac-deficient mice. J Clin Invest 2011; 121: 384–395.
Iida H, Towatari M, Iida M, Tanimoto M, Kodera Y, Ford AM et al. Protein expression and constitutive phosphorylation of hematopoietic transcription factors PU.1 and C/EBP beta in acute myeloid leukemia blasts. Int J Hematol 2000; 71: 153–158.
Negrotto S, Ng KP, Jankowska AM, Bodo J, Gopalan B, Guinta K et al. CpG methylation patterns and decitabine treatment response in acute myeloid leukemia cells and normal hematopoietic precursors. Leukemia 2012; 26: 244–254.
Ng KP, Ebrahem Q, Negrotto S, Mahfouz RZ, Link KA, Hu Z et al. p53 Independent epigenetic-differentiation treatment in xenotransplant models of acute myeloid leukemia. Leukemia 2011; 25: 1739–1750.
Saunthararajah Y, Maciejewski J . Polycomb segment myeloid malignancies. Blood 2012; 119: 1097–1098.
Sun W, Downing JR . Haploinsufficiency of AML1 results in a decrease in the number of LTR-HSCs while simultaneously inducing an increase in more mature progenitors. Blood 2004; 104: 3565–3572.
Theilgaard-Monch K, Jacobsen LC, Borup R, Rasmussen T, Bjerregaard MD, Nielsen FC et al. The transcriptional program of terminal granulocytic differentiation. Blood 2005; 105: 1785–1796.
Nakajima H, Ihle JN . Granulocyte colony-stimulating factor regulates myeloid differentiation through CCAAT/enhancer-binding protein epsilon. Blood 2001; 98: 897–905.
Truong BT, Lee YJ, Lodie TA, Park DJ, Perrotti D, Watanabe N et al. CCAAT/Enhancer binding proteins repress the leukemic phenotype of acute myeloid leukemia. Blood 2003; 101: 1141–1148.
Gery S, Gombart AF, Fung YK, Koeffler HP . C/EBPepsilon interacts with retinoblastoma and E2F1 during granulopoiesis. Blood 2004; 103: 828–835.
Nakajima H, Watanabe N, Shibata F, Kitamura T, Ikeda Y, Handa M . N-terminal region of CCAAT/enhancer-binding protein epsilon is critical for cell cycle arrest, apoptosis, and functional maturation during myeloid differentiation. J Biol Chem 2006; 281: 14494–14502.
Matsushita H, Nakajima H, Nakamura Y, Tsukamoto H, Tanaka Y, Jin G et al. C/EBPalpha and C/EBPvarepsilon induce the monocytic differentiation of myelomonocytic cells with the MLL-chimeric fusion gene. Oncogene 2008; 27: 6749–6760.
Wilson NK, Foster SD, Wang X, Knezevic K, Schutte J, Kaimakis P et al. Combinatorial transcriptional control in blood stem/progenitor cells: genome-wide analysis of ten major transcriptional regulators. Cell Stem Cell 2010; 7: 532–544.
Guo H, Ma O, Speck NA, Friedman AD . Runx1 deletion or dominant inhibition reduces Cebpa transcription via conserved promoter and distal enhancer sites to favor monopoiesis over granulopoiesis. Blood 2012; 119: 4408–4418.
Tonks A, Tonks AJ, Pearn L, Pearce L, Hoy T, Couzens S et al. Expression of AML1-ETO in human myelomonocytic cells selectively inhibits granulocytic differentiation and promotes their self-renewal. Leukemia 2004; 18: 1238–1245.
Dowdy CR, Frederick D, Zaidi SK, Colby JL, Lian JB, van Wijnen AJ et al. A germline point mutation in Runx1 uncouples its role in definitive hematopoiesis from differentiation. Exp Hematol (e-pub ahead of print 30 June 2013; doi:10.1016/j.exphem.2013.06.006).
Saunthararajah Y, Triozzi P, Rini B, Singh A, Radivoyevitch T, Sekeres M et al. p53-Independent, normal stem cell sparing epigenetic differentiation therapy for myeloid and other malignancies. Semin Oncol 2012; 39: 97–108.
Hu Z, Saunthararajah Y . CEBPE activation in PML-RARA cells by arsenic. Blood 2012; 119: 2177–2179.
Sexauer A, Perl A, Yang X, Borowitz M, Gocke C, Rajkhowa T et al. Terminal myeloid differentiation in vivo is induced by FLT3 inhibition in FLT3/ITD AML. Blood 2012; 120: 4205–4214.
Milhem M, Mahmud N, Lavelle D, Araki H, DeSimone J, Saunthararajah Y et al. Modification of hematopoietic stem cell fate by 5aza 2′ deoxycytidine and trichostatin A. Blood 2004; 103: 4102–4110.
Hu Z, Negrotto S, Gu X, Mahfouz R, Ng KP, Ebrahem Q et al. Decitabine maintains hematopoietic precursor self-renewal by preventing repression of stem cell genes by a differentiation-inducing stimulus. Mol Cancer Ther 2010; 9: 1536–1543.
Griswold IJ, Shen LJ, La Rosee P, Demehri S, Heinrich MC, Braziel RM et al. Effects of MLN518, a dual FLT3 and KIT inhibitor, on normal and malignant hematopoiesis. Blood 2004; 104: 2912–2918.
Acknowledgements
We gratefully acknowledge the gift of Runx1+/− mice from Jim Downing. YS is supported by grants from NIH (1R01CA138858), Department of Defense (PR081404) and Case Western Reserve University/Cleveland Clinic CTSA Grant Number UL1RR024989 from NIH/National Center for Research Resources (NCRR).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 3.0 Unported License. To view a copy of this license, visit http://creativecommons.org/licenses/by-nc-sa/3.0/
About this article
Cite this article
Ng, K., Hu, Z., Ebrahem, Q. et al. Runx1 deficiency permits granulocyte lineage commitment but impairs subsequent maturation. Oncogenesis 2, e78 (2013). https://doi.org/10.1038/oncsis.2013.41
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/oncsis.2013.41