Abstract
Bone metastasis is a prominent cause of morbidity and mortality in cancer. High rates of bone colonization in breast cancer, especially in the subtype expressing estrogen receptors (ERs), suggest tissue-specific proclivities for metastatic tumor formation. The mechanisms behind this subtype-specific organ-tropism remains largely elusive. Interestingly, as the major driver of ER+ breast cancer, ERs also have important roles in bone development and homeostasis. Thus, any agents targeting ER will also inevitably affect the microenvironment, which involves the osteoblasts and osteoclasts. Yet, how such microenvironmental effects are integrated with direct therapeutic responses of cancer cells remain poorly understood. Recent findings on ER mutations, especially their enrichment in bone metastasis, raised even more provocative questions on the role of ER in cancer–bone interaction. In this review, we evaluate the importance of ERs in bone metastasis and discuss new avenues of investigation for bone metastasis treatment based on current knowledge.
Similar content being viewed by others
Introduction
Steroidal and non-steroidal hormones regulate bone formation
Bone supports muscles and shapes vertebrates. Bone rigidity and strength are mostly derived from phosphate and calcium, which are the most enriched minerals during ossification. Multiple factors are involved in bone formation including hormones.1, 2 They are particularly important for bone development and remodeling in both male and female. For instance, parathyroid hormone (PTH) is critical for bone remodeling and calcium-level adjustment.3 Deficiencies in glucocorticoid, progesterone, androgen as well as estrogen often translate into severe pathological bone conditions.4 Sexual dimorphism usually affects hormonal responses between genders. Higher estrogen levels in females accounts for more assessable functions of estrogen receptors (ERs) in bone development and remodeling. Similarly, androgen receptors tend to contribute more to bone formation in males.5, 6 Reduced availability of estrogen negatively impacts bone mass and strength, which leads to higher risks of bone fractures in postmenopausal women.7, 8 To palliate osteoporosis, hormone replacement therapies consisting of estrogen alone or in combination with progesterone have been successfully used despite some side effects associated with higher risks of breast cancer development.9 Overall, a balanced hormonal production is sine qua non to maintaining healthy bones in both males and females.
Osteoprotective role of ERs
Estrogen treatment is well known to protect against bone loss. Most of this effect is mediated by ERs (ERα and ERβ), which are highly expressed in osteoblast and osteoclast lineages, suggesting protective functions of ERs in bone.2 Osteoblast-specific ERα-knockout mouse models showed loss of bone mass as well as strength.10 ERα is more expressed in cortical bone, which suggests predominant roles of the receptor in bone formation. This may also explain bone alterations observed in ERα-knockout mice.10 ERβ is highly expressed in trabecular bone cells, but very few studies have investigated the function of ERβ in bone development, remodeling and metastasis.11 Understanding the primary roles of ERα and ERβ in bone can open new opportunities to better target postmenopausal and cancer-induced bone loss.
Bone stromal cells construct and remodel the skeleton
The skeleton is a dynamic structure, which is constantly remodeled by several bone cell lineages. Osteoclasts are large multinucleated bone macrophages deriving from monocytes.12 They degrade bone matrix by creating acidic environments and secreting enzymes such as collagenases.12 These gaps are often refilled by mature mesenchymal cells called osteoblasts, which secrete bone matrixes to prevent bone loss.13 When osteoblasts stay embedded in the bone matrix, they differentiate into osteocytes that interconnect with each other. They are believed to have mechanosensory functions, which allow them to control bone remodeling.14 Hence, maintaining a good balance between osteoclast and osteoblast activity is primordial for healthy bone formation. Although, significant progress has been made to better understand the function of bone stromal cells during bone development and remodeling, their role in breast cancer bone metastasis still remains unclear. The finer appreciation of how bone stromal cells influence cancer dissemination may allow better prevention and treatment of bone metastasis.
Luminal breast cancers highly metastasize to the bone
Metastasis is the primary cause of cancer-related death. Cancer cells can migrate in group (collective migration) or individually (single-cell migration) to invade the stroma.15, 16 Single-cell cancer metastasis has been more scrutinized. It involves multiple steps including epithelial to mesenchymal transition (EMT), which allows invasion of blood vessels and lymphatic system (intravasation).3, 17 Circulating tumor cells can then migrate to other organs, extravasate and seek for hospitable environments with higher chances of survival.18, 19, 20 Disseminated tumor cells can stay latent for years before forming secondary tumors.17 Despite significant improvement in understanding the mechanism of cell dormancy, more research is needed before we can efficiently target latent cells.
Several types of cancer can metastasize to skeletal bone.21 Intriguingly, bone is the preferred metastatic niche for a few cancers including breast cancer.3, 17, 22 It is still unclear why such selectivity is seen for bone. It is plausible that bone offers a better microenvironment for tumor survival.23, 24 However, this answer may not be sufficient as breast cancer subtypes display different trends of migration to bone.25 Clinical evidence shows that luminal breast cancers have a higher selectivity for bone metastasis when compared with other breast cancer subtypes,25 such as hormone receptor-negative breast cancers, which tend to metastasize to visceral organs.26 Thus, factors intrinsic to breast tumor subtypes could determine their bone metastatic potential.
With the majority of breast cancer being luminal subtype, ERα has been the predominant targeted nuclear receptor for breast cancer treatment. To inhibit cancer progression, selective ER modulators, including tamoxifen have been commonly used. Although selective ER modulators antagonize ERα function in breast, they may also impede its activity in other tissues such as bone. The resulting bone loss observed in patients undergoing adjuvant therapy, strongly implies the need for functional ERs in bone. More importantly, such hormonal therapy may indirectly affect ERα-positive cancer progression via altering activities of osteoblasts and osteoclasts. However, this microenvironmental effect remains to be investigated.
In this review, we summarize advancements in bone development and remodeling in connection with bone metastasis, and highlight the role of ERs in both of these processes. Although the primary breast cancer targets, ERs, are significantly expressed in bone, their roles in bone metastasis is still largely equivocal. An integrative understanding of ERs in both bone and cancer cells will be a strong asset toward developing new approaches to prevent and cure metastasis, while maintaining the health and strength of skeletal bone.
ERs are necessary for bone development and remodeling
Estrogen and ERs in bone development
Steroid hormones such as estrogen are necessary for normal development of bone. Two main receptors, identified as ERα and ERβ, mediate the effects of estrogen. ERs are structurally highly similar, but they appear to have diverse functions.7, 10, 27 In vivo ER knockout mouse models clearly demonstrated that ERs were required for development and maintenance of reproductive organs.28 Considering the importance for estrogen for bone formation, it is plausible that ERs affect bone formation via their effects on ovaries, which are the primary sources of estrogen production in females. Along with reproductive organ alterations, many ER knockout mouse models exhibited bone alterations.29, 30 ERα and ERβ had opposite effects on longitudinal bone growth. ERβ knockout (ERβKO) mice had longer bone compare with ERα knockout (ERαKO) mice. Interestingly, double-knockout mice had an intermediate bone size, suggesting inhibitory roles of ERβ on ERα-induced bone growth. In his study, Lindberg did not find significant differences in trabecular bone mineral density (BMD) between ERαKO and ERβKO mice.30 However, other studies found that ERβKO mice had a higher trabecular BMD when compared with ERαKO mice.29, 31 Similarly, 12 months old ERβKO mice had higher BMD than wild-type mice in both cortical and trabecular bone.29 Overall, these results indicate important functions for ERβ in trabecular bone formation and suggest differential activities between ERα and ERβ in bone tissues (Figure 1a).
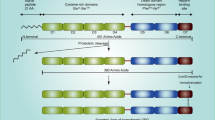
Differential expression of ERs in bone stromal cells. (a) Tissue-specific comparison of ERα (orange) and ERβ (blue) expression in trabecular bone versus cortical bone. (b) Shows osteoblast lineage (top), with high detection of ERα in mesenchymal stem cells (MSC) and pre-osteoclasts, but with decreased expression in mature osteoblasts and osteocytes. ERβ expression is maintained throughout the maturation cycle (middle). Disparate expression of ERs in females aged 40 or older (age>40) (bottom) showing expression of ERα in all cells except osteocytes and decreased levels of ERβ during osteoblast differentiation. (c) Osteoclast lineage (top) with ERα and ERβ expression during cell maturation (middle) and based on aging.
Mechanical loading also increases trabecular BMD in ERβKO but not in ERαKO, suggesting that ERβ may inhibit BMD in cancellous bone.32, 33 Inversely, bone stiffness was increased in female ERβKO mice as a consequence of enhanced periosteal formation under strain. This observation contrasts with ERαKO mice. In vitro studies found that osteoblasts derived from ERβKO periosteal bone can divide in response to mechanical strain, but not ERαKO osteoblasts. Despite possible redundancies between ERα and ERβ in bone, ER knockout mouse models have helped identify opposite functions between the two ER isoforms.32, 34, 35, 36 The predominance of either receptor during bone formation may be a determinant for the outcome.
ERs are expressed in bone stromal cells
ERα and ERβ are well expressed in bone tissues.37 Interestingly, Bord et al.33 reported a differential expression of ERs in osteoblast and osteoclast lineage cells according to bone histological studies. Although ERα was highly expressed in cortical bone, ERβ was predominant in trabecular bone, suggesting that they may have different functions in these tissues (Figure 1a). A change in ER expression was also observed during osteoclast and osteoblast maturation processes (Figures 1b and c). ERα detection was almost limited to pre-osteoclast and pre-osteoblast lineages as low expression was observed in mature cells. These results indicate that ERα may be involved in cell differentiation, but not required for the activity of mature osteoblasts and osteoclasts. In contrast to ERα, ERβ expression remained consistent throughout osteoblast differentiation, which strengthens the hypothesis that ERs have different roles during osteogenesis.
Aging also affects ER expression in bone (Figures 1b and c). Using human callus-derived biopsies, Batra et al.38 found that most bone stromal cells, including proliferative chondrocytes, were expressing both ER isoforms. No gender difference was observed between patients under age 40. However, in women close to menopause, both ERα and ERβ expression levels were considerably decreased in osteocytes. In osteoblasts and mesenchymal cells, while ERα remained constant, ERβ expression was lower. In men above age 40, ERβ expression rate was reduced only in mesenchymal cells.38 It is known that bone fracture repair is more challenging and requires more time in older individuals. This corroborates with age-related decrease of ER expression, suggesting possible involvement of ERs in bone repair processes. Yet, the mechanism of ER activity in bone metastasis remains to be determined.
Mechanisms involved in bone formation
Osteoclast lineage
Osteoclasts are bone macrophages responsible for bone resorption.39 Their activity is often increased under hypocalcemia to rescue blood calcium level. Several factors regulate osteoclastogenesis.12, 40, 41 Macrophage-colony stimulating factor induces expression of a receptor activator of nuclear factor kappa-B (RANK). RANK ligand (RANKL) and osteoprogeterin competitively bind RANK to promote and oppose osteoclastogenesis, respectively.42, 43, 44, 45 These ligands are mostly secreted by osteoblasts but may also derive from osteocytes and T cells.13 RANKL autocrine secretion by tumors has also been proposed, but it is still uncertain whether these ligands will be enough to activate osteolytic lesions.3 Activated RANK promotes nuclear factor kappa-B, as well as p38 mitogen-activated protein kinase signaling.46, 47 Downstream effectors such as nuclear factor of activated T cells, cytoplasmic, calcineurin-dependent 1 mediate transcription of tartrate-resistant acid phosphatase and cathepsin K.48, 49 These enzymes are crucial for bone resorption by osteoclasts. Others factors control osteoclast differentiation, which is based on fusion of multiple monocytes into mature osteoclasts. This differentiation process involves dendritic cell-specific transmembrane protein, a key transmembrane protein required for multinucleated osteoclast formation.50 Dendritic cell-specific transmembrane protein-depleted monocytes do not differentiate into mature osteoclasts, which often leads to osteopetrosis. In addition to osteoclast differentiation factors, estrogen was found to affect osteoclast survival through ERα. In fact, ERα-knockout mice had less apoptotic osteoclasts than control mice when estrogen was supplemented. The authors proposed that ERα was transcriptionally upregulating the pro-apoptotic factor Fas ligands in osteoclasts.31 Although the function of ERβ was not specifically addressed, this finding gave a better insight into osteoprotective roles of ERs in bones.
Osteoblast lineage
Osteoblasts have three different fates. They either undergo apoptosis, become lining cells or embed themselves in bone matrix. Osteoblast-specific ERα-depleted mice develop abnormal bone mass and strength.51 This observation stipulates that ERα osteoprotective effect may be mediated by its role in osteoblasts. Osteoblast maturation from mesenchymal stem cells is regulated by multiple factors some of which are Runt-related transcription factor 2, activating transcription factor 4, Osterix, Twist, activator protein 1 and specificity protein 3.52, 53
Several of these factors are involved in ER signaling. In fact, ERs often bind to activator protein 1 and Sp1 sites to regulate gene transcription in an ERE-independent manner.54 ERβ, in particular, was shown to regulate many genes without estrogen requirement through interaction with other transcription factors including nuclear factor kappa-B, Sp1, activator protein 1 and p53.55 Although the mechanism of ER transcriptional activity is not fully understood, it is possible that ERs regulate bone matrix formation by affecting key factors involved in osteoblast differentiation and maturation.
In vitro studies showed that ER activation could enhance osteoprogeterin and RANKL expression in estrogen-treated osteoblasts.56 More, the use of ER antagonist ICI-182,780 was able to abolish osteoprogeterin/RANKL production, indicating that ERs mediate their transcription.56 Further, the inhibitory effect of Twist in osteoblast differentiation negatively correlates with ERs function in bone matrix production.57 In fact, ERs are known to regulate Twist-dependent metastasis in cancer, as well as many other factors including Snail and Zeb1/2. These factors are highly involved in bone development, but also in EMT.58 Hence, it is possible that ERs effect on some of these EMT factors induces osteoblast differentiation, which promotes bone formation.
Literally, skeletal bone formation by osteoblasts involves a multistep procedure. Type 1 collagen is secreted along with other proteins including osteopontin (OPN) and osteocalcin to form the organic matrix called osteoid.59 Calcium–phosphate–hydroxide salt (hydroxyapatitematrix) is then added to osteoid allowing bone mineralization. Although this process of bone deposition may be highly influenced by ERs, the mechanisms involved have not been defined.
Osteocyte lineage
The majority of bone stromal cells are osteocytes, which derive from embedded osteoblasts in the bone matrix.60 These are long-lasting cells interacting with each other via cytoplasmic extensions. However, little is understood about osteocyte differentiation and maturation into neuron-like networks. So far, we know that matrix metalloproteinase 14, E11 antigen, dentin matrix protein 1, transforming growth factor (TGF)-beta inducible factor, osteoblast/osteocyte factor 45, Klotho and lysophosphatidic acid, are required for dendritic and canaliculi formation.61, 62, 63, 64, 65, 66 In addition, oxygen has a protective role in bone formation. Under mechanical strain, hypoxia-inducible factors 1α was strongly stabilized and found to inhibit anabolic signals in bone.67
Mechanical loading and unloading on bone also affect gene transcription in osteocytes, suggesting the hypothesis that osteocytes function as mechanosensors and may regulate bone remodeling.68, 69, 70 These results may also explain why physical activities maintain stronger and healthier bones.71, 72 With aging, decreased physical activity often promotes osteocytic senescence causing osteoporosis.73 Reduced osteocyte activity may be one of the drivers of osteopenia (bone loss) observed in spaceflight members. Despite all the evidence involving bone stromal cells in bone development and remodeling, we still do not know much about their mechanism of action. Dentrin matrix protein 1 regulates fibroblast growth factor 23, which is involved in phosphate metabolism in mature osteoblasts.61, 62 This is supported by clinical evidence showing autosomal recessive hypophosphatemia in patients with Dentrin matrix protein 1 loss-of-function mutations.62 Osteocytes can also inhibit Wnt signaling by inducing sclerostin that can bind the Wnt co-receptor LRP5/6, thereby opposing bone formation.74 Similarly, osteocytes can activate osteoclast formation through RANKL. We can stipulate from the above studies that osteocytes may serve as messengers between bone stromal cells to protect bone integrity. However, the mechanisms involved in osteocyte activity remain to be clarified.
ERs in breast cancer
Factors affecting ER status of breast cancer
More than 80% of breast cancers are positive for hormone receptor.75 Epidemiological studies have identified factors specifically associated with these breast cancer types, including female socioeconomic status, age, age at menarche, gravidity-parity, menopause, body habitus, exposure to exogenous hormones, BRCA mutation and breastfeeding.76, 77 In general, inverse associations between these factors and negative hormone receptor (ER−PR−) breast cancers are stronger than direct associations with positive hormone receptor subtypes. Higher socioeconomic status has been linked with a higher overall incidence of breast cancer, particularly those expressing ER and/or PR.77, 78 In regards to race, the highest prevalence of ER− breast cancer (to include the triple negative) has been demonstrated for African-American women, followed by Hispanics and Caucasians.77, 79, 80 Younger women more frequently develop ER− breast cancer.75, 81, 82, 83 Female body habitus assessed by body mass index was positively associated with the risk of developing ER+PR+ breast cancer; this relationship was significantly stronger for overweight and obese compared with normal-weight women.77, 84 Early age at menarche and late age at menopause have been shown to be independently associated with an increased incidence of ER+ and/or PR+ breast cancer subtypes.77, 85, 86 Nulliparity has been shown to increase the risk for hormone receptor-negative breast cancer.87 Parous women with more advanced age at first full-term pregnancy showed significantly higher prevalence of ER+/PR+ subtypes compared with their younger counterparts.88 Furthermore, having more children was associated with a lower risk of developing breast cancer expressing hormone receptors. The use of hormonal contraceptives (estrogen-progestin) was linked with a greater incidence of ER+ breast cancer; a similar but weaker association was observed for menopausal women using hormonal replacement therapy.89, 90 Women who are BRCA1 gene mutation carriers are at higher risk for developing ER−PR− breast cancer subtypes, whereas those with a BRCA2 gene mutation are more likely to have hormone receptor positive subtypes.91, 92
Among the factors affecting hormonal receptor status of breast cancer, lactation may deserve special consideration because of its potential implications to skeletal metastasis of breast cancer. Many studies have reported an inverse relationship between breastfeeding and the overall incidence of breast cancer, particularly with ER−PR− breast cancer.93 A recent meta-analysis of 27 distinct breast cancer studies corroborated a protective effect of breastfeeding against hormone receptor-negative breast cancers.94 Ever breastfeeding versus never breastfeeding in parous women was associated with a 10% risk reduction of developing ER−PR− breast cancers when adjusted for age, body mass index, number of full-term pregnancies and family history.94 This risk reduction was even twice as strong for the triple-negative breast cancers. Furthermore, the length of breastfeeding demonstrated a dose-response inverse relationship with the incidence of hormone receptor-negative breast cancers.94 Women who breastfed for a combined duration of 2 years or more in their lifetime have a significant reduction of developing ER−PR− breast cancers, particularly before menopause. Despite a natural link of lactation with parity, there is an independent 4% reduction in breast cancer risk for every 12 months of breastfeeding, in addition to a 7% reduction for each full-term pregnancy.95
The mechanisms by which lactation affects ER−PR− breast cancer subtypes remain unclear. Altered exposure to endogenous hormones, such as low estrogen/progesterone and higher androgen levels, during the process can suppress ER expression, thereby selectively promoting ER− cancer cell induction and/or proliferation.96 Nonhormonal mechanisms, including changes in the immune system, alterations in cellular communication, adhesion and apoptosis may also have a role. Furthermore, irreversible changes in the breasts that take place upon lactation and the effects of breast milk within the ducts may provide protective effects against cancer through the cellular and molecular maturation and/or involution of the breast tissue.97
Mammary gland and bone homeostasis share common factors
Many factors involved in mammary gland formation and EMT also associate with bone homeostasis and remodeling. For instance, RANK/RANKL signaling, which promotes osteoclast differentiation in bone, has been found to have a major role in lobuloalveolar development during pregnancy.98 RANK-knockout mice develop highly apoptotic mammary epithelia associated with R-spondin-mediated inaction of Wnt signaling, which prevents lobuloalveologenesis.98 Mice overexpressing RANK had increased epithelial cell proliferation, less apoptosis and impaired differentiation of lobuloalveolar structures.99 Importantly, transient increased expression of RANKL during pregnancy promotes mouse mammary stem cell proliferation, which correlates with higher pregnancy-related breast cancer incidence.100 RANKL depletion can also inhibit tumor formation and reduce bone metastasis in mice.101 Further, in BRCA1-deficient mouse model, RANKL inhibition with denosumab considerably reduces tumor growth.102 Beside RANK/RANKL, vitamin D receptor, which protects from osteoporosis, was shown to attenuate mammary gland formation and may contribute to post-weaning mammary gland regression.103, 104, 105 Tumor-suppressive functions of vitamin D have also been proposed in breast cancer.106
Other main factors involved in bone formation are also essential for mammary development. Indeed, the expression of a key regulator of osteogenesis, Runt-related transcription factor 2, in developing mammary epithelial cells was found to induce OPN during lactation.107, 108 Mammary-specific OPN-depleted mice had lactation deficiencies because of lower alveolar structures suggesting a determinant role of OPN in olveologenesis.109 Further, OPN expression was drastically increased in spontaneous mammary tumors in c-MYC transgenic mice and OPN expression was found to associate with metastasis.110 Another factor known as Calcitonin, is involved in calcium blood calcium regulation and inhibits osteoclast activity in bone.111 Basically, Calcitonin opposes PTH activity. Therefore, in response to hypercalcemia, thyroid cells release calcitonin to inhibit bone resorption. Intriguingly, breast paracrine production of calcitonin increases during pregnancy but quickly drops before parturition, which may imply a local protective role of calcitonin against calcification.112 Further, clinical data reveal decreased calcitonin expression in metastatic breast cancer.113 These observations suggest commonality of factors involved in bone and breast development.
A connection between lactation and skeletal metastasis of breast cancer has not been addressed in the literature. As breastfeeding protects from breast cancer overall, particularly from ER−PR− subtypes, proportionally more ER+ cancer types are represented by this group. Furthermore, as ER+ breast cancer subtypes tend to metastasize to bone more frequently compared with the ER− counterparts, which commonly target the visceral organs,26 lactation may indirectly implicate skeletal breast cancer dissemination. Lactation is stimulated by prolactin, a hormone released from the anterior pituitary gland already during pregnancy. This contribute to a rapid bone turnover in lactating women because of increased osteoblastic and osteoclastic activities.114, 115 During breastfeeding, nerves within the nipples stimulated by suckling connect with the central neural system and suppress the gonadotropin-releasing hormone in the hypothalamus, which culminates in a decline of circulating estradiol. This estrogen deficiency results in postpartum amenorrhea,116 and contributes to bone loss similar to menopause. Interestingly, the profound bone loss during lactation cannot solely be attributed to low estrogens.117, 118 Lactating breasts secrete PTH-related protein (PTHrP) into systemic circulation,119 and PTHrP blood levels correlate with the bone resorption markers and lactation-induced total bone loss.120 Low estrogen levels appear to synergize the PTHrP-related bone loss during lactation. Actually, lactation is the only nonmalignant state in which PTHrP is present in the circulation.121 However, these phenomena are followed by fast recoveries upon weaning, indicating potential crosstalk between breast and bone. This observation associates with high mobilization of calcium for milk production knowing that nursing mothers secrete 300–400 mg of calcium into milk daily.118 The physiologic levels of PTHrP during lactation are controlled the calcium-sensing receptor, the master regulator of systemic calcium metabolism. Activation of the calcium-sensing receptor in lactating breasts downregulates PTHrP, and increases calcium transport into milk.120, 122 Interestingly, breast cancer utilizes exactly these mechanisms to invade, colonize and destroy bone, but the expression of calcium-sensing receptor in breast cancer cells upregulates PTHrP production.123 Perhaps these molecular connections between mammary gland functions and bone homeostasis are part of the mechanisms underlying bone tropisms of breast cancer, especially the ER+ subtypes.124 It can be speculated that mammary epithelial cells may preferentially survive and proliferate in a foreign environment with similar molecular and ionic properties.
ERα promotes tumor growth
ERα is the primary target for breast cancer treatment. Approximately 75% of breast cancers are ERα positive.88, 125 Upon estrogen binding, ERs dimerize and translocate to the nucleus where they regulate transcription of target genes in a ERE dependent or independent manner.126, 127, 128 ERα induces cell growth by activating multiple growth factors and inhibiting tumor suppressors.127, 129 Hormonal therapy inhibits ERα activity and can significantly enhance survival of patients.115, 130 Unfortunately, resistance often occurs, leading to cancer recurrence. Recurrent tumors develop new properties, which makes them more resistant to therapeutic treatments.131 After years of controversy, we now know that the ESR1 gene can acquire mutations that confer further resistance in breast cancer tumors.132, 133, 134
The ESR1 mutations found in metastatic breast cancer patients appear to cluster in a mutational ‘hot-spot’ surrounding residues 536–538 in the hormone binding domain, resulting in constitutive activation of the receptor. The most frequent ESR1 ‘hot-spot’ mutations in metastatic breast cancer to date are the E380Q, Y537N, Y537S and D538G somatic alterations. These mutations appear to be selected by aromatase inhibitor treatment,135 and in some genetic backgrounds also confer resistance to the antiestrogen tamoxifen.136 However, clinical evidence suggests that some of the ESR1 mutations are sensitive to fulvestrant, and/or everolimus, or palbociclib treatment, thus these agents are useful in metastatic mutation-positive patients.137, 138 Recent pre-clinical studies suggest that the newer orally available selective ER degrader drugs, such as AZD9496 and GDC-0810, are also very effective in reducing tumor growth of ESR1 mutant models.139, 140 Thus, patients with ESR1 mutations can be treated with our currently approved, and highly efficacious targeted therapeutic regimens for ER+ breast cancer.
Using next-generation sequencing, approximately 20% of metastatic patients have acquired ESR1 mutations, whereas the frequency of these mutations in primary invasive breast cancers is low.141 It is now apparent that clinical monitoring of ESR1 mutations in circulating cell-free DNA in ER+ cancer patients is a feasible and very sensitive method to detect acquired mutations, especially in metastatic breast cancer.142 Using cell-free DNA coupled with sensitive digital drop PCR methods, the frequency of ESR1 mutations in metastatic patients is now estimated to be between 30 and 50% in breast cancer.143 Importantly, ESR1 circulating mutations in the blood are independent risk factors for poor outcome after aromatase inhibitor treatment failure.144 Circulating ESR1 mutations are also frequently detectable before clinical progression is observed, thus cell-free DNA ESR1 mutant assays may prove useful for earlier interventional studies and change of treatment decisions in metastatic patients. Further studies are warranted to determine if these liquid biopsy assays for ESR1 mutations will also prove to be useful predictive factors to guide the treatment of patients with metastatic breast cancer. Another important clinical question is whether these mutations have a role in metastatic behavior, and whether they may directly influence tumor progression in addition to conferring hormone resistance.145 Hopefully, ongoing pre-clinical studies will provide the answers to this critical question.
Recently, accumulation of ESR1 mutations upon aromatase treatment has been observed especially in bone metastatic tumors, suggesting a selective role of bone microenvironment for such mutations.135, 146, 147 Alas, there is not enough evidence to support the role of ER mutations in bone.
ERs regulate EMT
EMT-driving factors are often critical regulators in developmental biology and pathological conditions. As such, TGFβ, hypoxia, Notch, Wnt, bone morphogenetic protein (BMP), matrix metalloproteinases, platelet-derived growth factor, PTHrP, vascular endothelial growth factor, epithelial growth factor receptor, interleukins (IL-6, IL-8, IL-11 and IL-1), catepsin K, and αvβ3 integrin, which are involved in EMT and mesenchymal to epithelial transition processes in breast cancer are also found in the bone microenvironment. EMT-regulated genes, such as E-cadherin, N-cadherin, Zeb1/2, vimentin, mir200, snail, slug and twist1, are crucial for bone metastasis.58, 148, 149, 150 Interestingly, ERs regulate most of these factors. ERα was shown to affect E-cadherin expression in cells such as MCF7 where knocking down the receptor induced loss of E-cadherin expression.151, 152 However, this property can be lost in advance stages of breast cancer.153 Intriguingly, the ERβ isoform displays strong regulatory effects on EMT/mesenchymal to epithelial transition factors and metastasis in breast cancer. ERβ expression was enough to induce E-cadherin expression in basal-like ERα-negative cells, suggesting anti-metastatic functions of the receptor in breast cancer.154, 155 Despite significant progress, the role of ERs in bone metastasis is still uncertain.
Pre-metastatic niche in bone
A new concept of pre-metastatic bone niche has recently emerged. Primary tumors may instigate the formation of distant pre-metastatic lesions by activating bone stromal cells to secrete various chemokines, cytokines and other factors. For instance, C-X-C motif chemokine 12 (CXCL12), insulin-like growth factor, bone morphogenetic protein, tumor necrosis factor alpha, matrix metalloproteinases, TGFβ, chemokine (C-C motif) ligand 2, colony stimulating factor 1, semaphorin 3A and vascular endothelial growth factor A were expressed upon development of primary tumor.156, 157 Further, Lysyl oxidase secreted from hypoxic primary tumors was found to accumulate at pre-metastatic sites of distant organs and promote the recruitment of bone marrow-derived cells.158 CD11b+ myeloid cells were found to mediate this effect through secretion of metalloproteinases-2.158 The extracellular matrix proteoglycan versican activates macrophages through the Toll-like receptor complexes (TLR2 and TLR6) to maintain pro-metastatic inflamed micro-environments.159 Interestingly, tumor-derived exosomes can educate bone marrow progenitors located at distant metastatic niches through the tyrosine receptor kinase MET.160 Further, mir-122 secretion from primary tumors inhibits glucose metabolism of distant-niche cells, thereby increasing nutrient availability at these pre-metastatic niches.161 The survival promoting effect of this observation implies critical roles of glucose metabolism for disseminated tumor survival, and it may be of great interest to elicit how glucose influences cell awakening from dormancy, especially in the context of bone metastasis. The vascular endothelial growth factor receptors 1 is also involved in pre-metastatic niche vascularization, which may allow circulating tumors to reach tissue-specific sites.162, 163 Although all these factors prepare hospitable niches for future metastatic cells, it is still unclear whether disseminating tumors choose their niche or whether pre-metastatic niches are the one choosing their ‘guests’. Perhaps both options matter considering all obstacles tumors have to overcome to reach metastatic sites.
Luminal cancer dormancy in bone
Little is known about dormancy of ER+ cancer cells in the bone,164 mainly due to the lack of pre-clinical models. Nevertheless, several recent studies have significantly advanced our understanding of dormancy mechanisms in other systems,165 which may also be applicable to ER+ breast cancer. In particular, it has become increasingly evident that dormant and viable metastatic cells are often found adjacent to blood vessels in the ‘peri-vascular niche’.166 Mechanistically, crosstalk between endothelial cells and cancer cells via thrombospondin-1-mediated signaling may keep cancer cells quiescent. Recently, IL-6 cytokine leukemia inhibitory factor receptor, as well as signal transducer and activator of transcription 3, were found to promote dormancy states in breast cancer cells disseminated in bone.67 Immunosurveillance by natural killer cells may help to reinforce the dormancy of peri-vascular cancer cells by eliminating those that re-enter cell cycle.167 How do cancer cell survive during the prolonged dormancy period? Along with others, we have previously found that c-SRC is a key factor in ER+ cell colonization of bone. The proto-oncogene tyrosine-protein kinase c-SRC promotes bone metastasis and survival by activating AKT/mammalian target of rapamycin signaling in response to C-X-C motif chemokine 12 binding to CXCR4.168 It is noticeable that c-SRC also promotes estrogen independence in ER+ cells,62 thus linking survival in dormancy to therapeutic resistance. In addition, C-X-C motif chemokine 12 and CXCR4 signaling may also be responsible to retain cancer cells in the peri-vascular niche.169 Taken together, these findings suggest a signaling network that dictates the dormancy behavior of cancer cells in the bone marrow.
ERs in early cancer arousal in bone
Some dormant cancer cells are eventually activated and resume aggressive growth to become overt metastases. Our understanding of this process is equally scarce especially in ER+ tumors. It has been observed that proliferating cancer cells often target special structures lining the inner side of bones called endosteal that are enriched in osteoblasts and their precursors, which is designated as the ‘osteogenic niche’.169, 170 The fate of the tumor cell is in part determined by their ability to interact with osteoblasts through cell–cell contact proteins such CXCR4, E-cadherin, annexin II receptor, AXL receptor, IL-6.171 In particular, the heterotypic adhesion junctions between the E-cadherin of cancer cells and N-cadherin of osteogenic cells can activate the downstream mammalian target of rapamycin signaling in cancer cells and trigger proliferation.170 The interaction between ERα with these pathways172 suggest a role of ER in metastatic cancer re-activation within the osteogenic niche. However, the direct interaction with osteogenic cells may not be sufficient, and the re-activation may be an integrated result of other cellular components of the niche, and the availability of cytokines and growth factors produced from the niche,173 including estrogen. Indeed, Ogba et al.174 recently observed that estrogen could trigger tumor revival from dormancy. In an elegant study, it is demonstrated soluble VCAM1 may be secreted by cancer cells including ER+ ones to recruit activated osteoclasts.175 These findings demonstrate the involvement of osteoclasts in full activation of dormant cancer cells. It is possible that there are distinct stages of the re-activation process. In an earlier stage, the osteogenic niche drives initial proliferation of cancer cells via direct cell–cell interaction and paracrine mechanisms. This process may take a long period of time until osteoclasts are recruited and foster a faster progression of micrometastases.
ERs in osteolytic vicious cycle
Metastasized breast cancer often drives osteolytic lesions, which are due to increased bone resorption as a result of unbalanced activities between bone stromal cells. This process of bone resorption releases multiple factors such as insulin-like growth factor, fibroblast growth factor and substantial amount of TGF-β, which are often stored in the bone matrix.176 TGF-β induces secretion of paracrine factors including PTHrP and IL-11, which promote osteoclast maturation. In addition, cancer cells express tumor necrosis factor alpha, chemokine (C-C motif) ligand 2, soluble intercellular adhesion molecule 1, soluble vascular cell adhesion molecule 1, matrix metalloproteinases and Jagged 1, which foster more osteoclastogenesis.177 This will perpetuate malicious crosstalk between degenerating bones and growing tumors thereby promoting bone loss. Clinical data suggest a strong association between bone loss prevention and decreased bone metastasis in postmenopausal, but not premenopausal women, indicating a role of ERs in bone metastasis.178 Further, ERα-positive cancer cells had almost fivefold increased bone colonization in ovariectomized mice when compared with control mice.179, 180 These results suggest a protective role of estrogen in bone, but the role of ERs remains to be clarified (Figure 2).
Role of ERs in bone metastasis. ERα is a predominant driver of primary tumor formation from normal mammary glands. ERα regulates several EMT factors to drive metastasis but this seems to require E2. Some of these metastatic effects may be attributed to ER mutations. Circulating tumors often disseminate to bone and form micro-metastatic niches by interacting with osteoblasts. Factors such as IL-6 cytokine leukemia inhibitory factor (LIF) promotes cell dormancy. Several other factors involved in cell-cell adhesion (cadherins and integrins) may be ER regulated. Increased bone macro-metastases following E2 treatment suggests a role of ERs in tumor re-activation and growth.
Clinical implication of ER and current therapeutic options
The majority of luminal cancers display bone metastasis often accompanied with intense bone pain and pathological fractures.181 Bone pain management may require complex treatment especially when neurologic pains are involved.182 Breast metastatic tumors are known to induce bone resorption as bone fractures considerably increase. Factors involved in breast cancer treatment may also contribute to rapid bone loss in patients. Therapies such as ovariectomy or aromatase inhibitor treatment including letrozole and exemestane promote bone weakening because of the inhibition of estrogen-dependent ER activity.183 Ultimately, these cancers often become resistant to hormonal treatment. Many of the recurrent tumors develop ER-independent survival mechanism or acquire ER mutations, which make the receptor constitutively active.
New therapies are being investigated to reduce side effects of ER inhibition on bones. One solution has been the use of selective estrogen modulators with less deleterious effect on bones. For instance, tamoxifen exhibits some bone protective roles, which contrast with its anti-estrogenic activity in breast. In contrast, the selective ER degrader fulvestrant induces ER degradation in both bone and breast, leading to bone weakening, which may significantly increase the risks of bone metastasis and promote cancer progression. The use of these therapies in clinic makes imminent the need to elicit the mechanisms underlying ER ligand effects in bone with regard to ER isoforms as both ERα and ERβ represent potent targets mediating ER signaling in bone (Figure 1). More recently, a new class of selective ER modulator/selective ER degrader hybrid drugs developed to reduce side effects and increase efficacy in breast cancer, showed ER agonist effects in bone.184 Another way to reduce ER-dependent bone loss has been the association of anti-resorptive drugs such as bisphosphonates to hormonal therapy. Bisphosphonates decrease bone loss by inhibiting osteoclastogenesis and inducing cell death. More anabolic drugs, such as teriparatide, are also used for their potential to rescue bone loss. Although these drugs are well used, the clinical outcome of patients can significantly improve if we get a better understanding of their mode of action.
Conclusion and perspectives
The mineralized structure of skeleton makes bone research more challenging. Despite these limitations, tremendous efforts have been made to better understand normal bone biology as well as malignancies in bone. One important family of molecules that we have not fully understood in the bone context are the ERs. Osteoblast and osteoclast lineage cells clearly depend on estrogen to be fully functional. ER knockout mouse models have helped identified some similarities, but mostly divergent functions between ERα and ERβ in bone formation. Intriguingly, mammary glands are also highly regulated by ERs and cancer cells derived from breast primary tumors incline to metastasize to bone. Systemic hormone therapies have been implemented to treat ER+ breast cancer before and after they form bone metastasis. Although such therapies are often effective, bone metastasis remains incurable and usually develops resistance. Numerous pre-clinical and clinical studies have been performed to understand endocrine resistant mechanisms. However, only a few of them were done in the context of bone and bone metastases. As a result, the impact of endocrine therapies on osteoclasts and osteoblasts, two cell types that intimately interact with cancer cells during bone colonization, has been largely ignored. Studies are urgently needed to synthesize both microenvironmental and cancer-intrinsic effects of endocrine therapies on bone niche formation, cancer cell survival and metastatic tumor growth (Figure 3).
Effect of antiestrogen therapies on bone turnover. Estrogen (E2) promotes bone formation by opposing osteoclastogenesis and enhancing osteoblast activity. Breast cancer therapies affect bone metabolism and may impact bone metastasis. Aromatase inhibitors prevent E2 production, which may leads to more bone loss because of increased osteoclast activity. Fulvestrant alters E2 signaling by inducing degradation of ERs, which leads decreased bone formation.
We are entering a new era of ESR1 mutations and we are just beginning to perceive the contribution of mutant ESR1 to cancer progression and drug resistance. Interestingly, a recent study suggests that bone metastasis patients accumulate ESR1 mutations in their circulating DNAs. Moreover, aromatase inhibitors but not tamoxifen appear to enrich ESR1 mutations.135 Understanding the mechanisms underlying these observations will undoubtedly shed light on distinct roles of ERs in bone and cancer cells during bone colonization and development of endocrine resistance. Thanks to significant progress in imaging technologies and new tool developments, we may soon answer some essential questions in bone metastasis. We believe that genetic dissection of microenvironmental and cancerous ERs at different stages of bone metastasis may allow us to better apprehend the molecular mechanisms of ERs in bone colonization, and may open new opportunities for the development of new anti-metastatic therapies.
References
Imai Y, Youn M-Y, Inoue K, Takada I, Kouzmenko A, Kato S . Nuclear receptors in bone physiology and diseases. Physiol Rev 2013; 93: 481–523.
Long F . Building strong bones: molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol 2011; 13: 27–38.
Mundy GR . Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer 2002; 2: 584–593.
Zhou H, Cooper MS, Seibel MJ . Endogenous glucocorticoids and bone. Bone Res 2013; 1: 107–119.
Manolagas SC, O’Brien Ca, Almeida M . The role of estrogen and androgen receptors in bone health and disease. Nat Rev Endocrinol 2013; 9: 699–712.
Sims Na, Dupont S, Krust a, Clement-Lacroix P, Minet D, Resche-Rigon M et al. Deletion of estrogen receptors reveals a regulatory role for estrogen receptors-beta in bone remodeling in females but not in males. Bone 2002; 30: 18–25.
Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002; 288: 321–333.
Ahlborg HG, Johnell O, Turner CH, Rannevik G, Karlsson MK . Bone loss and bone size after menopause. N Engl J Med 2003; 349: 327–334.
Reed ML, Merriam GR, Kargi AY . Adult growth hormone deficiency - benefits, side effects, and risks of growth hormone replacement. Front Endocrinol (Lausanne) 2013; 4: 64.
Khalid AB, Krum SA . Estrogen receptors alpha and beta in bone. Bone 2016; 87: 130–135.
Braidman IP, Hainey L, Batra G, Selby PL, Saunders PT, Hoyland Ja . Localization of estrogen receptor beta protein expression in adult human bone. J Bone Miner Res 2001; 16: 214–220.
Boyle WJ, Simonet WS, Lacey DL . Osteoclast differentiation and activation. Nature 2003; 423: 337–342.
Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ . Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev 1999; 20: 345–357.
O’Brien CA, Nakashima T, Takayanagi H . Osteocyte control of osteoclastogenesis. Bone 2013; 54: 258–263.
Friedl P, Gilmour D . Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol 2009; 10: 445–457.
Clark AG, Vignjevic DM . Modes of cancer cell invasion and the role of the microenvironment. Curr Opin Cell Biol 2015; 36: 13–22.
Futakuchi M, Fukamachi K, Suzui M . Heterogeneity of tumor cells in the bone microenvironment: mechanisms and therapeutic targets for bone metastasis of prostate or breast cancer. Adv Drug Deliv Rev 2016; 99: 206–211.
Brien CAO, Pollett A, Gallinger S, Dick JE, O’Brien Ca . A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 2007; 445: 106–110.
Yang ZF, Ho DW, Ng MN, Lau CK, Yu WC, Ngai P et al. Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell 2008; 13: 153–166.
Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C et al. Identification and expansion of human colon-cancer-initiating cells. Nature 2007; 445: 111–115.
Coleman RE . Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res 2006; 12: 6243s–6249s.
Wu S, Sun J, Yang L, Tang L, Wang X . Patterns of distant metastasis in Chinese women according to breast cancer subtypes. Oncotarget 2016; 7: 47975–47984.
Yoneda T, Hiraga T . Crosstalk between cancer cells and bone microenvironment in bone metastasis. Biochem Biophys Res Commun 2005; 328: 679–687.
Yoneda T, Williams PJ, Hiraga T, Niewolna M, Nishimura R . A bone-seeking clone exhibits different biological properties from the MDA-MB-231 parental human breast cancer cells and a brain-seeking clone in vivo and in vitro. J Bone Miner Res 2001; 16: 1486–1495.
Yeung C, Hilton J, Clemons M, Mazzarello S, Hutton B, Haggar F et al. Estrogen, progesterone, and HER2/neu receptor discordance between primary and metastatic breast tumours? A review. Cancer Metastasis Rev 2016; 35: 427–437.
Lee SJ, Park S, Ahn HK, Yi JH, Cho EY, Sun JM et al. Implications of bone-only metastases in breast cancer: favorable preference with excellent outcomes of hormone receptor positive breast cancer. Cancer Res Treat 2011; 43: 89–95.
Deroo BJ, Korach KS, Barros RPa, Gustafsson J-AJ-Å a, Brzozowski AM, Pike AC et al. Estrogen receptors and human disease. Nature 2014; 116: 561–570.
Walker VR, Korach KS . Estrogen receptor knockout mice as a model for endocrine research. ILAR J 2004; 45: 455–461.
Windahl SH, Hollberg K, Vidal O, Gustafsson JA, Ohlsson C, Andersson G . Female estrogen receptor β-/- mice are partially protected against age-related trabecular bone loss. J Bone Min Res 2001; 16: 1388–1398.
Lindberg MK, Alatalo SL, Halleen JM, Mohan S, Gustafsson J-Å, Ohlsson C . Estrogen receptor specificy in the regulation of the skeleton in femal mice. J Endocrinol 2001; 171: 229–236.
Nakamura T, Imai Y, Matsumoto T, Sato S, Takeuchi K, Igarashi K et al. Estrogen prevents bone loss via estrogen receptor α and induction of Fas ligand in osteoclasts. Cell 2007; 130: 811–823.
Saxon LK, Galea G, Meakin L, Price J, Lanyon LE . Estrogen receptors α and β have different gender-dependent effects on the adaptive responses to load bearing in cancellous and cortical bone. Endocrinology 2012; 153: 2254–2266.
Bord S, Horner A, Beavan S, Compston J . Estrogen receptors alpha and beta are differentially expressed in developing human bone. J Clin Endocrinol Metab 2001; 86: 2309–2314.
Windahl SH, Vidal O, Andersson G, Gustafsson Ja, Ohlsson C . Increased cortical bone mineral content but unchanged trabecular bone mineral density in female ERbeta(-/-) mice. J Clin Invest 1999; 104: 895–901.
Vidal O, Lindberg M, Sävendahl L, Lubahn DB, Ritzen EM, Gustafsson JA et al. Disproportional body growth in female estrogen receptor-α-inactivated mice. Biochem Biophys Res Commun 1999; 265: 569–571.
Galea GL, Meakin LB, Sugiyama T, Zebda N, Sunters A, Taipaleenmaki H et al. Estrogen receptor α mediates proliferation of osteoblastic cells stimulated by estrogen and mechanical strain, but their acute down-regulation of the Wnt antagonist Sost is mediated by estrogen receptor β. J Biol Chem 2013; 288: 9035–9048.
Imamura T, Sugiyama T, Kusuhara S . Expression and localization of estrogen receptors α and β mRNA in medullary bone of laying hens. Anim Sci J 2006; 77: 223–229.
Batra GS, Hainey L, Freemont AJ, Andrew G, Saunders PTK, Hoyland JA et al. Evidence for cell-specific changes with age in expression of oestrogen receptor (ER) α and β in bone fractures from men and women. J Pathol 2003; 200: 65–73.
Wynn TA, Chawla A, Pollard JW . Macrophage biology in development, homeostasis and disease. Nature 2013; 496: 445–455.
Wada T, Nakashima T, Hiroshi N, Penninger JM . RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med 2006; 12: 17–25.
Kagiya T . MicroRNAs and osteolytic bone metastasis: the roles of microRNAs in tumor-induced osteoclast differentiation. J Clin Med 2015; 4: 1741–1752.
Yasuda H, Shima N, Nakagawa N, Mochizuki SI, Yano K, Fujise N et al. Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology 1998; 139: 1329–1337.
Simonet W, Lacey D, Dunstan C, Kelley M, Chang M-S, Lüthy R et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 1997; 89: 309–319.
Wittrant Y, Théoleyre S, Chipoy C, Padrines M, Blanchard F, Heymann D et al. RANKL/RANK/OPG: new therapeutic targets in bone tumours and associated osteolysis. Biochim Biophys Acta Rev Cancer 2004; 1704: 49–57.
Dougall WC . Molecular pathways: osteoclast-dependent and osteoclast-independent roles of the RANKL/RANK/OPG pathway in tumorigenesis and metastasis. Clin Cancer Res 2012; 18: 326–335.
Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature 1999; 402: 304–309.
Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 1999; 397: 315–323.
Koga T, Matsui Y, Asagiri M, Kodama T, de Crombrugghe B, Nakashima K et al. NFAT and osterix cooperatively regulate bone formation. Nat Med 2005; 11: 880–885.
Takayanagi H . Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nat Rev 2007; 7: 292–304.
Yagi M, Miyamoto T, Sawatani Y, Iwamoto K, Hosogane N, Fujita N et al. DC-STAMP is essential for cell-cell fusion in osteoclasts and foreign body giant cells. J Exp Med 2005; 202: 345–351.
Melville KM, Kelly NH, Khan Sa, Schimenti JC, Ross FP, Main RP et al. Female mice lacking estrogen receptor-alpha in osteoblasts have compromised bone mass and strength. J Bone Min Res 2014; 29: 370–379.
Karsenty G, Kronenberg HM, Settembre C . Genetic control of bone formation. Annu Rev Cell Dev Biol 2009; 25: 629–648.
Qi HL, Aguiar DJ, Williams SM, La Pean A, Pan W, Verfaillie CM . Identification of genes responsible for osteoblast differentiation from human mesodermal progenitor cells. Proc Natl Acad Sci USA 2003; 100: 3305–3310.
Carroll JS, Meyer Ca, Song J, Li W, Geistlinger TR, Eeckhoute J et al. Genome-wide analysis of estrogen receptor binding sites. Nat Genet 2006; 38: 1289–1297.
Vivar OI, Zhao X, Saunier EF, Griffin C, Mayba OS, Tagliaferri M et al. Estrogen receptor β binds to and regulates three distinct classes of target genes. J Biol Chem 2010; 285: 22059–22066.
Bord S, Ireland DC, Beavan SR, Compston JE . The effects of estrogen on osteoprotegerin, RANKL, and estrogen receptor expression in human osteoblasts. Bone 2003; 32: 136–141.
Bialek P, Kern B, Yang X, Schrock M, Sosic D, Hong N et al. A twist code determines the onset of osteoblast differentiation. Dev Cell 2004; 6: 423–435.
Zeisberg M, Neilson EG . Biomarkers for epithelial-mesenchymal transitions. J Clin Invest 2009; 119: 1429–1437.
Karsenty G . How many factors are required to remodel bone? Nat Med 2000; 6: 970–971.
Manolagas SC . Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 2000; 21: 115–137.
Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B et al. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet 2006; 38: 1310–1315.
Lorenz-Depiereux B, Bastepe M, Benet-Pagès A, Amyere M, Wagenstaller J, Müller-Barth U et al. DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat Genet 2006; 38: 1248–1250.
Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K et al. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 2006; 444: 770–774.
Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ et al. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med 2011; 17: 1231–1234.
Chan KM, Wong HLX, Jin G, Liu B, Cao R, Cao Y et al. MT1-MMP inactivates ADAM9 to regulate FGFR2 signaling and calvarial osteogenesis. Dev Cell 2012; 22: 1176–1190.
Prideaux M, Staines KA, Jones ER, Riley GP, Pitsillides AA, Farquharson C . MMP and TIMP temporal gene expression during osteocytogenesis. Gene Expr Patterns 2015; 18: 29–36.
Johnson RW, Finger EC, Olcina MM, Vilalta M, Aguilera T, Miao Y et al. Induction of LIFR confers a dormancy phenotype in breast cancer cells disseminated to the bone marrow. Nat Cell Biol 2016; 18: 1260.
Nampei A, Hashimoto J, Hayashida K, Tsuboi H, Shi K, Tsuji I et al. Matrix extracellular phosphoglycoprotein (MEPE) is highly expressed in osteocytes in human bone. J Bone Miner Metab 2004; 22: 176–184.
Gluhak-Heinrich J, Ye L, Bonewald LF, Feng JQ, MacDougall M, Harris SE et al. Mechanical loading stimulates dentin matrix protein 1 (DMP1) expression in osteocytes in vivo. J Bone Miner Res 2003; 18: 807–817.
Rubin CT . Skeletal strain and the functional significance of bone architecture. Calcif Tissue Int 1984; 36: S11–S18.
Maïmoun L, Sultan C . Effects of physical activity on bone remodeling. Metabolism 2011; 60: 373–388.
Tan VP, Macdonald HM, Kim S, Nettlefold L, Gabel L, Ashe MC et al. Influence of physical activity on bone strength in children and adolescents: a systematic review and narrative synthesis. J Bone Miner Res 2014; 29: 2161–2181.
Svejme O, Ahlborg HG, Karlsson MK . Physical activity reduces bone loss in the distal forearm in post-menopausal women - a 25-year prospective study. Scand J Med Sci Sport 2014; 24: 159–165.
Cui Y, Niziolek PJ, MacDonald BT, Zylstra CR, Alenina N, Robinson DR et al. Lrp5 functions in bone to regulate bone mass. Nat Med 2011; 17: 684–691.
Howlader N, Altekruse SF, Li CI, Chen VW, Clarke CA, Ries LA et al. US incidence of breast cancer subtypes defined by joint hormone receptor and HER2 status. J Natl Cancer Inst 2014; 106: 1–8.
Althuis M, Fergenbaum J, Garcia-Closas M, Brinton L, Madigan M, Sherman M . Etiology of hormone receptor-defined breast cancer: a systematic review of the literature. Cancer Epidemiol Biomarkers Prev 2004; 13: 1558–1568.
Setiawan VW, Monroe KR, Wilkens LR, Kolonel LN, Pike MC, Henderson BE . Breast cancer risk factors defined by estrogen and progesterone receptor status: the multiethnic cohort study. Am J Epidemiol 2009; 169: 1251–1259.
Akinyemiju TF, Pisu M, Waterbor JW, Altekruse SF . Socioeconomic status and incidence of breast cancer by hormone receptor subtype. Springerplus 2015; 4: 508.
Amend K, Hicks D, Ambrosone CB . Breast cancer in African-American women: differences in tumor biology from European-American women. Cancer Res 2006; 66: 8327–8330.
Stark A, Kleer CG, Martin I, Awuah B, Nsiah-Asare A, Takyi V et al. African ancestry and higher prevalence of triple-negative breast cancer: findings from an international study. Cancer 2010; 116: 4926–4932.
Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K et al. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA 2006; 295: 2492–2502.
Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V . Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California Cancer Registry. Cancer 2007; 109: 1721–1728.
Stead LA, Lash TL, Sobieraj JE, Chi DD, Westrup JL, Charlot M et al. Triple-negative breast cancers are increased in black women regardless of age or body mass index. Breast Cancer Res 2009; 11: R18.
Suzuki R, Rylander-Rudqvist T, Ye W, Saji S, Wolk A . Body weight and postmenopausal breast cancer risk defined by estrogen and progesterone receptor status among Swedish women: a prospective cohort study. Int J Cancer 2006; 119: 1683–1689.
La Vecchia C, Negri E, Franceschi S, Parazzini F . Long-term impact of reproductive factors on cancer risk. Int J Cancer 1993; 53: 215–219.
Chlebowski RT, Anderson GL, Lane DS, Aragaki AK, Rohan T, Yasmeen S et al. Predicting risk of breast cancer in postmenopausal women by hormone receptor status. J Natl Cancer Inst 2007; 99: 1695–1705.
Press DJ, Pharoah P . Risk factors for breast cancer: a reanalysis of two case-control studies from 1926 and 1931. Epidemiology 2010; 21: 566–572.
Aktipis CA, Ellis BJ, Nishimura KK, Hiatt RA . Modern reproductive patterns associated with estrogen receptor positive but not negative breast cancer susceptibility. Evol Med Public Heal 2014; 2015: 52–74 eou028+.
Chen WY, Hankinson SE, Schnitt SJ, Rosner BA, Holmes MD, Colditz GA . Association of hormone replacement therapy to estrogen and progesterone receptor status in invasive breast carcinoma. Cancer 2004; 101: 1490–1500.
Hwang ES, Chew T, Shiboski S, Farren G, Benz CC, Wrensch M . Risk factors for estrogen receptor-positive breast cancer. Arch Surg 2005; 140: 58–62.
Pan H, He Z, Ling L, Ding Q, Chen L, Zha X et al. Reproductive factors and breast cancer risk among BRCA1 or BRCA2 mutation carriers: results from ten studies. Cancer Epidemiol 2014; 38: 1–8.
Friebel TM, Domchek SM, Rebbeck TR . Modifiers of cancer risk in BRCA1 and BRCA2 mutation carriers: systematic review and meta-analysis. J Natl Cancer Inst 2014; 106: 1–15.
Faupel-Badger JM, Arcaro KF, Balkam JJ, Heather Eliassen A, Hassiotou F, Lebrilla CB et al. Postpartum remodeling, lactation, and breast cancer risk: summary of a national cancer institute-sponsored workshop. J Natl Cancer Inst 2013; 105: 166–174.
Islami F, Liu Y, Jemal A, Zhou J, Weiderpass E, Colditz G et al. Breastfeeding and breast cancer risk by receptor status-a systematic review and meta-analysis. Ann Oncol 2015; 26: 2398–2407.
Collaborative Group on Hormonal Factors in Breast Cancer CG on HF in B Cancer CG on HF in B Cancer CG on HF in B Cancer CG on HF in B, Vessey M, Baron J et al. Breast cancer and breastfeeding: collaborative reanalysis of individual data from 47 epidemiological studies in 30 countries, including 50302 women with breast cancer and 96973 women without the disease. Lancet (London, England) 2002; 360: 187–195.
McNamara KM, Yoda T, Takagi K, Miki Y, Suzuki T, Sasano H . Androgen receptor in triple negative breast cancer. J Steroid Biochem Mol Biol 2013; 133: 66–76.
Kobayashi S, Sugiura H, Ando Y, Shiraki N, Yanagi T, Yamashita H et al. Reproductive history and breast cancer risk. Breast Cancer 2012; 19: 302–308.
Joshi PA, Waterhouse PD, Kannan N, Narala S, Fang H, Di Grappa MA et al. RANK signaling amplifies WNT-responsive mammary progenitors through R-SPONDIN1. Stem Cell Rep 2015; 5: 31–44.
Gonzalez-Suarez E, Branstetter D, Armstrong A, Dinh H, Blumberg H, Dougall WC . RANK overexpression in transgenic mice with mouse mammary tumor virus promoter-controlled RANK increases proliferation and impairs alveolar differentiation in the mammary epithelia and disrupts lumen formation in cultured epithelial acini. Mol Cell Biol 2007; 27: 1442–1454.
Asselin-Labat M-L, Vaillant F, Sheridan JM, Pal B, Wu D, Simpson ER et al. Control of mammary stem cell function by steroid hormone signalling. Nature 2010; 465: 798–802.
Canon JR, Roudier M, Bryant R, Morony S, Stolina M, Kostenuik PJ et al. Inhibition of RANKL blocks skeletal tumor progression and improves survival in a mouse model of breast cancer bone metastasis. Clin Exp Metastasis 2008; 25: 119–129.
Nolan E, Vaillant F, Branstetter D, Pal B, Giner G, Whitehead L et al. RANK ligand as a potential target for breast cancer prevention in BRCA1-mutation carriers. Nat Med 2016; 22: 933–939.
Zinser GM, Welsh J . Accelerated mammary gland development during pregnancy and delayed postlactational involution in vitamin D3 receptor null mice. Mol Endocrinol 2007; 18: 2208–2223.
Lips P, Van Schoor NM . The effect of vitamin D on bone and osteoporosis. Best Pract Res Clin Endocrinol Metab 2011; 25: 585–591.
Welsh J . Targets of vitamin D receptor signaling in the mammary gland. J Bone Miner Res 2007; 22: V86–V90.
Lopes N, Paredes J, Costa JL, Ylstra B, Schmitt F . Vitamin D and the mammary gland: a review on its role in normal development and breast cancer. Breast Cancer Res 2012; 14: 211.
Rodrigues LR, Teixeira Ja, Schmitt FL, Paulsson M, Lindmark-Mänsson H . The role of osteopontin in tumor progression and metastasis in breast cancer. Cancer Epidemiol Biomarkers Prev 2007; 16: 1087–1097.
Shore P . A role for Runx2 in normal mammary gland and breast cancer bone metastasis. J Cell Biochem 2005; 96: 484–489.
Nemir M, Bhattacharyya D, Li X, Singh K, Mukherjee AB, Mukherjee BB . Targeted inhibition of osteopontin expression in the mammary gland causes abnormal morphogenesis and lactation deficiency. J Biol Chem 2000; 275: 969–976.
Rittling SR, Novick ER . Osteopontin expression in mammary gland development and tumorigenesis. Cell Growth Differ 1997; 8: 1061–1069.
Keller J, Catala-Lehnen P, Huebner AK, Jeschke A, Heckt T, Lueth A et al. Calcitonin controls bone formation by inhibiting the release of sphingosine 1-phosphate from osteoclasts. Nat Commun 2014; 5: 5215.
Tverberg LA, Gustafson MF, Scott TL, Arzumanova IV, Provost ER, Yan AW et al. Induction of calcitonin and calcitonin receptor expression in rat mammary tissue during pregnancy. Endocrinology 2000; 141: 3696–3702.
Wang X, Nakamura M, Mori I, Takeda K, Nakamura Y, Utsunomiya H et al. Calcitonin receptor gene and breast cancer: quantitative analysis with laser capture microdissection. Breast Cancer Res Treat 2004; 83: 109–117.
Carneiro RM, Prebehalla L, Tedesco MB, Sereika SM, Hugo M, Hollis BW et al. Lactation and bone turnover: a conundrum of marked bone loss in the setting of coupled bone turnover. J Clin Endocrinol Metab 2010; 95: 1767–1776.
Fisher B, Dignam J, Bryant J, DeCillis a, Wickerham DL, Wolmark N et al. Five versus more than five years of tamoxifen therapy for breast cancer patients with negative lymph nodes and estrogen receptor-positive tumors. J Natl Cancer Inst 1996; 88: 1529–1542.
McNEILLY AS, CCK TAY, GLASIER A . Physiological mechanisms underlying lactational amenorrhea. Ann N Y Acad Sci 1994; 709: 145–155.
Funk JL, Shoback DM, Genant HK . Transient osteoporosis of the hip in pregnancy : natural history of changes in bone mineral density. Clin Endocrinol (Oxf) 1995; 43: 373–382.
Kovacs CS, Kronenberg HM . Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr Rev 1997; 18: 832–872.
Sowers MF, Hollis BW, Shapiro B, Randolph J, Janney Ca, Zhang D et al. Elevated parathyroid hormone-related peptide associated with lactation and bone density loss. JAMA 1996; 276: 549–554.
Vanhouten JN, Wysolmerski JJ . Low estrogen and high parathyroid hormone-related peptide levels contribute to accelerated bone resorption and bone loss in lactating mice. Endocrinology 2003; 144: 5521–5529.
Wysolmerski JJ . Interactions between breast, bone, and brain regulate mineral and skeletal metabolism during lactation. Ann N Y Acad Sci 2010;; 1192: 161–169.
Vanhouten JN, Wysolmerski JJ . The calcium-sensing receptor in the breast. Best Pract Res Clin Endocrinol Metab 2013;; 27: 403–414.
Sanders JL, Chattopadhyay N, Kifor O, Yamaguchi T, Butters RR, Brown EM . Extracellular calcium-sensing receptor expression and its potential role in regulating parathyroid hormone-related peptide secretion in human breast cancer cell lines. Endocrinology 2000; 141: 4357–4364.
Fontanella C, Fanotto V, Rihawi K, Aprile G, Puglisi F . Skeletal metastases from breast cancer: pathogenesis of bone tropism and treatment strategy. Clin Exp Metastasis 2015; 32: 819–833.
Anderson WF, Chatterjee N, Ershler WB, Brawley OW . Estrogen receptor breast cancer phenotypes in the Surveillance, Epidemiology, and End Results database. Breast Cancer Res Treat 2002; 76: 27–36.
McDevitt MA, Glidewell-Kenney C, Jimenez MA, Ahearn PC, Weiss J, Jameson JL et al. New insights into the classical and non-classical actions of estrogen: evidence from estrogen receptor knock-out and knock-in mice. Mol Cell Endocrinol 2008; 290: 24–30.
Marino M, Galluzzo P, Ascenzi P . Estrogen signaling multiple pathways to impact gene transcription. Curr Genomics 2006; 7: 497–508.
Björnström L, Sjöberg M . Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol Endocrinol 2005; 19: 833–842.
Welboren W-J, Stunnenberg HG, Sweep FCGJ, Span PN . Identifying estrogen receptor target genes. Mol Oncol 2007; 1: 138–143.
Coombes RC, Hall E, Gibson LJ, Paridaens R, Jassem J, Delozier T et al. A randomized trial of exemestane after two to three years of tamoxifen therapy in postmenopausal women with primary breast cancer. N Engl J Med 2004; 350: 1081–1092.
Jeselsohn R, Buchwalter G, De Angelis C, Brown M, Schiff R . ESR1 mutations—a mechanism for acquired endocrine resistance in breast cancer. Nat Rev Clin Oncol 2015; 12: 1–11.
Fuqua SAW, Gu G, Rechoum Y . Estrogen receptor (ER) α mutations in breast cancer: hidden in plain sight. Breast Cancer Res Treat 2014; 144: 11–19.
Thomas C, Gustafsson J-Å . Estrogen receptor mutations and functional consequences for breast cancer. Trends Endocrinol Metab 2015; 26: 467–476.
Barone I, Brusco L, Fuqua SaW . Estrogen receptor mutations and changes in downstream gene expression and signaling. Clin Cancer Res 2010; 16: 2702–2708.
Schiavon G, Hrebien S, Garcia-Murillas I, Cutts RJ, Pearson a, Tarazona N et al. Analysis of ESR1 mutation in circulating tumor DNA demonstrates evolution during therapy for metastatic breast cancer. Sci Transl Med 2015; 7: 313ra182.
Gelsomino L, Gu G, Rechoum Y, Beyer AR, Pejerrey SM, Tsimelzon A et al. ESR1 mutations affect anti-proliferative responses to tamoxifen through enhanced cross-talk with IGF signaling. Breast Cancer Res Treat 2016; 157: 253–265.
Fribbens C, O’Leary B, Kilburn L, Hrebien S, Garcia-Murillas I, Beaney M et al. Plasma ESR1 Mutations and the treatment of estrogen receptor-positive advanced breast cancer. J Clin Oncol 2016; 34: 2961–2968.
Spoerke JM, Gendreau S, Walter K, Qiu J, Wilson TR, Savage H et al. Heterogeneity and clinical significance of ESR1 mutations in ER-positive metastatic breast cancer patients receiving fulvestrant. Nat Commun 2016; 7: 11579.
Weir HM, Bradbury RH, Rabow AA, Buttar D, Callis RJ, Curwen JO et al. AZD9496: an oral estrogen receptor inhibitor that blocks the growth of ER-positive and ESR1-mutant breast tumors in preclinical models. Cancer Res 2016; 76: 3307–3318.
Joseph JD, Darimont B, Zhou W, Arrazate A, Young A, Ingalla E et al. The selective estrogen receptor downregulator GDC-0810 is efficacious in diverse models of ER+ breast cancer. Elife 2016; 5: 1–34.
Gu G, Fuqua SAW . ESR1 mutations in breast cancer: proof-of-concept challenges clinical action. Clin Cancer Res 2016; 22: 1034–1036.
Chu D, Paoletti C, Gersch C, VanDenBerg DA, Zabransky DJ, Cochran RL et al. ESR1 mutations in circulating plasma tumor DNA from metastatic breast cancer patients. Clin Cancer Res 2016; 22: 993–999.
Takeshita T, Yamamoto Y, Yamamoto-Ibusuki M, Inao T, Sueta A, Fujiwara S et al. Clinical significance of monitoring ESR1 mutations in circulating cell-free DNA in estrogen receptor positive breast cancer patients. Oncotarget 2016; 7: 32504–32518.
Clatot F, Perdrix A, Augusto L, Beaussire L, Calbrix C, Sefrioui D et al. Kinetics, prognostic and predictive values of ESR1 circulating mutations in metastatic breast cancer patients progressing on aromatase inhibitor. Oncotarget 2016; 7: 74448–74459.
Fuqua SA . The role of estrogen receptors in breast cancer metastasis. J Mammary Gland Biol Neoplasia 2001; 6: 407–417.
Alluri PG, Speers C, Chinnaiyan AM . Estrogen receptor mutations and their role in breast cancer progression. Breast Cancer Res 2014; 16: 494.
Ma CX, Reinert T, Chmielewska I, Ellis MJ . Mechanisms of aromatase inhibitor resistance. Nat Rev Cancer 2015; 15: 261–275.
Mock K, Preca B-T, Brummer T, Brabletz S, Stemmler MP, Brabletz T . The EMT-activator ZEB1 induces bone metastasis associated genes including BMP-inhibitors. Oncotarget 2015; 6: 14399–14412.
Ottewell PD, O’Donnell L, Holen I . Molecular alterations that drive breast cancer metastasis to bone. Bonekey Rep 2015; 4: 643.
Sethi S, Macoska J, Chen W, Sarkar FH . Molecular signature of epithelial-mesenchymal transition (EMT) in human prostate cancer bone metastasis. Am J Transl Res 2010; 3: 90–99.
Bouris P, Skandalis SS, Piperigkou Z, Afratis N, Karamanou K, Aletras AJ et al. Estrogen receptor alpha mediates epithelial to mesenchymal transition, expression of specific matrix effectors and functional properties of breast cancer cells. Matrix Biol 2015; 43: 42–60.
Ye Y, Xiao Y, Wang W, Yearsley K, Gao JX, Shetuni B et al. ERalpha signaling through slug regulates E-cadherin and EMT. Oncogene 2010; 29: 1451–1462.
Guttilla IK, Adams BD, White BA . ERα, microRNAs, and the epithelial-mesenchymal transition in breast cancer. Trends Endocrinol Metab 2012; 23: 73–82.
Bado I, Nikolos F, Rajapaksa G, Gustafsson J-åke . ERβ decreases the invasiveness of triple-negative breast cancer cells by regulating mutant p53 oncogenic function. Oncotarget 2016; 7: 19–21.
Mak P, Leav I, Pursell B, Bae D, Yang X, Taglienti CA et al. ERβ impedes prostate cancer EMT by destabilizing HIF-1α and inhibiting VEGF-mediated Snail nuclear localization: implications for Gleason grading. Cancer Cell 2010; 17: 319–332.
Weidle UH, Birzele F, Kollmorgen G, Ruger R . Molecular mechanisms of bone metastasis. Cancer Genomics Proteomics 2016; 13: 1–12.
Kitamura T, Qian B-Z, Pollard JW . Immune cell promotion of metastasis. Nat Rev Immunol 2015; 15: 73–86.
Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell 2009; 15: 35–44.
Kim S, Takahashi H, Lin W-W, Descargues P, Grivennikov S, Kim Y et al. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature 2009; 457: 102–106.
Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med 2012; 18: 883–891.
Fong MY, Zhou W, Liu L, Alontaga AY, Chandra M, Ashby J et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat Cell Biol 2015; 17: 183–194.
Kaplan RN, Riba RD, Zacharoulis S, Anna H, Vincent L, Costa C et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 2005; 438: 820–827.
Grunewald M, Avraham I, Dor Y, Bachar-Lustig E, Itin A, Yung S et al. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell 2006; 124: 175–189.
Zhang XH-F, Giuliano M, Trivedi MV, Schiff R, Osborne CK . Metastasis dormancy in estrogen receptor-positive breast cancer. Clin Cancer Res 2013; 19: 6389–6397.
Sosa MS, Bragado P, Aguirre-Ghiso JA . Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer 2014; 14: 611–622.
Ghajar CM, Peinado H, Mori H, Matei IR, Evason KJ, Brazier H et al. The perivascular niche regulates breast tumour dormancy. Nat Cell Biol 2013; 15: 807–817.
Malladi S, MacAlinao DG, Jin X, He L, Basnet H, Zou Y et al. Metastatic latency and immune evasion through autocrine inhibition of WNT. Cell 2016; 165: 45–60.
Zhang XHF, Wang Q, Gerald W, Hudis CA, Norton L, Smid M et al. Latent bone metastasis in breast cancer tied to Src-dependent survival signals. Cancer Cell 2009; 16: 67–78.
Price TT, Burness ML, Sivan A, Warner MJ, Cheng R, Lee CH et al. Dormant breast cancer micrometastases reside in specific bone marrow niches that regulate their transit to and from bone. Sci Transl Med 2016; 8: 340ra73-340ra73.
Wang H, Yu C, Gao X, Welte T, Muscarella AM, Tian L et al. The osteogenic niche promotes early-stage bone colonization of disseminated breast cancer cells. Cancer Cell 2015; 27: 193–210.
Ursini-Siegel J, Siegel PM . The influence of the pre-metastatic niche on breast cancer metastasis. Cancer Lett 2016; 380: 281–288.
Svoronos N, Perales-Puchalt A, Allegrezza MJ, Rutkowski MR, Payne KK, Tesone AJ et al. Tumor cell-independent estrogen signaling drives disease progression through mobilization of myeloid-derived supressor cells. Cancer Discov 2016; 7: 1–14.
Croucher PI, McDonald MM, Martin TJ . Bone metastasis: the importance of the neighbourhood. Nat Rev Cancer 2016; 16: 373–386.
Ogba N, Manning NG, Bliesner BS, Ambler SK, Haughian JM, Pinto MP et al. Luminal breast cancer metastases and tumor arousal from dormancy are promoted by direct actions of estradiol and progesterone on the malignant cells. Breast Cancer Res 2014; 16: 489.
Lu X, Mu E, Wei Y, Riethdorf S, Yang Q, Yuan M et al. VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging α4β1-positive osteoclast progenitors. Cancer Cell 2011; 20: 701–714.
Ignatiadis M, Sotiriou C . Luminal breast cancer: from biology to treatment. Nat Rev Clin Oncol 2013; 10: 494–506.
Massagué J, Obenauf AC . Metastatic colonization by circulating tumour cells. Nature 2016; 529: 298–306.
Coleman RE, Marshall H, Cameron D, Dodwell D, Burkinshaw R, Keane M et al. Breast-cancer adjuvant therapy with zoledronic acid. N Engl J Med 2011; 365: 1–10.
Ottewell PD, Wang N, Brown HK, Reeves KJ, Fowles CA, Croucher PI et al. Zoledronic acid has differential antitumor activity in the pre- and postmenopausal bone microenvironment in vivo. Clin Cancer Res 2014; 20: 2922–2932.
Holen I, Walker M, Nutter F, Fowles A, Evans CA, Eaton CL et al. Oestrogen receptor positive breast cancer metastasis to bone: inhibition by targeting the bone microenvironment in vivo. Clin Exp Metastasis 2016; 33: 211–224.
Shenoy PA, Kuo A, Vetter I, Smith MT . The walker 256 breast cancer cell-induced bone pain model in rats. Front Pharmacol 2016; 7: 1–13.
Buga S, Sarria JE . The management of pain in metastatic bone disease. Cancer Control 2012; 19: 154–166.
Oesterreich S, Henry NL, Kidwell KM, Van Poznak CH, Skaar TC, Dantzer J et al. Associations between genetic variants and the effect of letrozole and exemestane on bone mass and bone turnover. Breast Cancer Res Treat 2015; 154: 263–273.
Wardell SE, Nelson ER, Chao CA, McDonnell DP . Bazedoxifene exhibits antiestrogenic activity in animal models of tamoxifen-resistant breast cancer: implications for treatment of advanced disease. Clin Cancer Res 2013; 19: 2420–2431.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Bado, I., Gugala, Z., Fuqua, S. et al. Estrogen receptors in breast and bone: from virtue of remodeling to vileness of metastasis. Oncogene 36, 4527–4537 (2017). https://doi.org/10.1038/onc.2017.94
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2017.94
This article is cited by
-
Management of bone loss due to endocrine therapy during cancer treatment
Osteoporosis International (2023)
-
Serum biomarker-based osteoporosis risk prediction and the systemic effects of Trifolium pratense ethanolic extract in a postmenopausal model
Chinese Medicine (2022)
-
Critical illness and bone metabolism: where are we now and what is next?
European Journal of Medical Research (2022)
-
Estrogen receptor (ESR1) mutation in bone metastases from breast cancer
Modern Pathology (2018)