Abstract
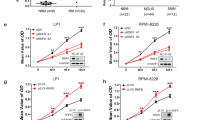
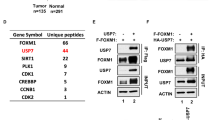
We previously reported that PU.1 is downregulated in the majority of myeloma cell lines and primary myeloma cells of certain myeloma patients, and conditional expression of PU.1 in such myeloma cell lines induced cell cycle arrest and apoptosis. We found downregulation of IRF4 protein in the U266 myeloma cell line following induction of PU.1. Previous studies reported that knockdown of IRF4 in myeloma cell lines induces apoptosis, prompting us to further investigate the role of IRF4 downregulation in PU.1-induced cell cycle arrest and apoptosis in myeloma cells. PU.1 induced downregulation of IRF4 at the protein level, cell cycle arrest and apoptosis in six myeloma cell lines. Chromatin immunoprecipitation (ChIP) revealed that PU.1 directly binds to the IRF4 promoter, whereas a reporter assay showed that PU.1 may suppress IRF4 promoter activity. Stable expression of IRF4 in myeloma cells expressing PU.1 partially rescued the cells from apoptosis induced by PU.1. As it was reported that IRF4 directly binds to the IRF7 promoter and downregulates its expression in activated B cell-like subtype of diffuse large B cell lymphoma cells, we performed ChIP assays and found that IRF4 directly binds the IRF7 promoter in myeloma cells. It is known that IRF7 positively upregulates interferon-β (IFNβ) and induces apoptosis in many cell types. Binding of IRF4 to the IRF7 promoter decreased following PU.1 induction, accompanied by downregulation of IRF4 protein expression. Knockdown of IRF7 protected PU.1-expressing myeloma cells from apoptosis. Furthermore, IFNβ, which is a downstream target of IRF7, was upregulated in myeloma cells along with IRF7 after PU.1 induction. Finally, we evaluated the mRNA expression levels of PU.1, IRF4 and IRF7 in primary myeloma cells from patients and found that PU.1 and IRF7 were strongly downregulated in contrast to the high expression levels of IRF4. These data strongly suggest that PU.1-induced apoptosis in myeloma cells is associated with IRF4 downregulation and subsequent IRF7 upregulation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout













Similar content being viewed by others
References
Kyle R, Rajkumar S . Multiple myeloma. Blood 2008; 111: 2962–2972.
Palumbo A, Anderson K . Multiple myeloma. N Engl J Med 2011; 364: 1046–1060.
Raab M, Podar K, Breitkreutz I, Richardson P, Anderson K . Multiple myeloma. Lancet 2009; 374: 324–339.
Ocio E, Mateos M, Maiso P, Pandiella A, San-Miguel J . New drugs in multiple myeloma: mechanisms of action and phase I/II clinical findings. Lancet Oncol 2008; 9: 1157–1165.
Moreau P, Richardson P, Cavo M, Orlowski R, San Miguel J, Palumbo A et al. Proteasome inhibitors in multiple myeloma: 10 years later. Blood 2012; 120: 947–959.
Palumbo A, Facon T, Sonneveld P, Blade J, Offidani M, Gay F et al. Thalidomide for treatment of multiple myeloma: 10 years later. Blood 2008; 111: 3968–3977.
Palumbo A, Rajkumar S, San Miguel J, Larocca A, Niesvizky R, Morgan G et al. International myeloma working group consensus statement for the management, treatment, and supportive care of patients with myeloma not eligible for standard autologous stem-cell transplantation. J Clin Oncol 2014; 32: 587–600.
Chapman MA, Lawrence MS, Keats JJ, Cibulskis K, Sougnez C, Schinzel AC et al. Initial genome sequencing and analysis of multiple myeloma. Nature 2011; 471: 467–472.
Klemsz MJ, McKercher SR, Celada A, Van Beveren C, Maki RA . The macrophage and B cell-specific transcription factor PU.1 is related to the ETS oncogene. Cell 1990; 61: 113–124.
McKercher SR, Torbett BE, Anderson KL, Henkel GW, Vestal DJ, Baribault H et al. Targeted disruption of the PU.1 gene results in multiple hematopoietic abnormalities. EMBO J 1996; 15: 5647–5658.
Kaufman RM, Pham CT, Ley TJ . Transgenic analysis of a 100- kb human beta-globin cluster-containing DNA fragment propagated as a bacterial artificial chromosome [published erratum appears in Blood 2000; 95(3): 744]. Blood 1999; 94: 3178–3184.
Li Y, Okuno Y, Zhang P, Radomska HS, Chen H, Iwasaki H et al. Regulation of the PU.1 gene by distal elements. Blood 2001; 98: 2958–2965.
Okuno Y, Huang G, Rosenbauer F, Evans EK, Radomska HS, Iwasaki H et al. Potential autoregulation of transcription factor PU.1 by an upstream regulatory element. Mol Cell Biol 2005; 25: 2832–2845.
Okuno Y, Huettner CS, Radomska HS, Petkova V, Iwasaki H, Akashi K et al. Distal elements are critical for human CD34 expression in vivo. Blood 2002; 100: 4420–4426.
Okuno Y, Iwasaki H, Huettner CS, Radomska HS, Gonzalez DA, Tenen DG et al. Differential regulation of the human and murine CD34 genes in hematopoietic stem cells. Proc Natl Acad Sci USA 2002; 99: 6246–6251.
Rosenbauer F, Wagner K, Kutok JL, Iwasaki H, Le Beau MM, Okuno Y et al. Acute myeloid leukemia induced by graded reduction of a lineage-specific transcription factor, PU.1. Nat Genet 2004; 36: 624–630.
Rosenbauer F, Owens BM, Yu L, Tumang JR, Steidl U, Kutok JL et al. Lymphoid cell growth and transformation are suppressed by a key regulatory element of the gene encoding PU.1. Nat Genet 2006; 38: 27–37.
Tatetsu H, Ueno S, Hata H, Yamada Y, Takeya M, Mitsuya H et al. Down-regulation of PU.1 by methylation of distal regulatory elements and the promoter is required for myeloma cell growth. Cancer Res 2007; 67: 5328–5336.
Ueno S, Tatetsu H, Hata H, Iino T, Niiro H, Akashi K et al. PU.1 induces apoptosis in myeloma cells through direct transactivation of TRAIL. Oncogene 2009; 28: 4116–4125.
Yuki H, Ueno S, Tatetsu H, Niiro H, Iino T, Endo S et al. PU.1 is a potent tumor suppressor in classical Hodgkin lymphoma cells. Blood 2013; 121: 962–970.
Shaffer AL, Emre NC, Lamy L, Ngo VN, Wright G, Xiao W et al. IRF4 addiction in multiple myeloma. Nature 2008; 454: 226–231.
Kronke J, Udeshi ND, Narla A, Grauman P, Hurst SN, McConkey M et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 2014; 343: 301–305.
Lu G, Middleton RE, Sun H, Naniong M, Ott CJ, Mitsiades CS et al. The myeloma drug lenalidomide promotes the cereblon-dependent destruction of Ikaros proteins. Science 2014; 343: 305–309.
Zhu Y, Braggio E, Shi C, Kortuem K, Bruins L, Schmidt J et al. Identification of cereblon-binding proteins and relationship with response and survival after IMiDs in multiple myeloma. Blood 2014; 124: 536–545.
Yang Y, Shaffer AL 3rd, Emre NC, Ceribelli M, Zhang M, Wright G et al. Exploiting synthetic lethality for the therapy of ABC diffuse large B cell lymphoma. Cancer Cell 2012; 21: 723–737.
Chen Q, Gong B, Mahmoud-Ahmed AS, Zhou A, Hsi ED, Hussein M et al. Apo2L/TRAIL and Bcl-2-related proteins regulate type I interferon-induced apoptosis in multiple myeloma. Blood 2001; 98: 2183–2192.
Tian E, Zhan F, Walker R, Rasmussen E, Ma Y, Barlogie B et al. The role of the Wnt-signaling antagonist DKK1 in the development of osteolytic lesions in multiple myeloma. N Engl J Med 2003; 349: 2483–2494.
Desai S, Bolick SC, Maurin M, Wright KL . PU.1 regulates positive regulatory domain I-binding factor 1/Blimp-1 transcription in lymphoma cells. J Immunol 2009; 183: 5778–5787.
Nelsen B, Tian G, Erman B, Gregoire J, Maki R, Graves B et al. Regulation of lymphoid-specific immunoglobulin mu heavy chain gene enhancer by ETS-domain proteins. Science 1993; 261: 82–86.
Nikolajczyk BS, Sanchez JA, Sen R . ETS protein-dependent accessibility changes at the immunoglobulin mu heavy chain enhancer. Immunity 1999; 11: 11–20.
Ochiai K, Maienschein-Cline M, Simonetti G, Chen J, Rosenthal R, Brink R et al. Transcriptional regulation of germinal center B and plasma cell fates by dynamical control of IRF4. Immunity 2013; 38: 918–929.
Piya S, Moon AR, Song PI, Hiscott J, Lin R, Seol DW et al. Suppression of IRF4 by IRF1, 3, and 7 in Noxa expression is a necessary event for IFN-gamma-mediated tumor elimination. Mol Cancer Res 2011; 9: 1356–1365.
Mamane Y, Heylbroeck C, Genin P, Algarte M, Servant M, LePage C et al. Interferon regulatory factors: the next generation. Gene 1999; 237: 1–14.
Ozato K, Tailor P, Kubota T . The interferon regulatory factor family in host defense: mechanism of action. J Biol Chem 2007; 282: 20065–20069.
Takaoka A, Tamura T, Taniguchi T . Interferon regulatory factor family of transcription factors and regulation of oncogenesis. Cancer Sci 2008; 99: 467–478.
Honda K, Yanai H, Negishi H, Asagiri M, Sato M, Mizutani T et al. IRF-7 is the master regulator of type-I interferon-dependent immune responses. Nature 2005; 434: 772–777.
Schonheit J, Kuhl C, Gebhardt ML, Klett FF, Riemke P, Scheller M et al. PU.1 level-directed chromatin structure remodeling at the Irf8 gene drives dendritic cell commitment. Cell Rep 2013; 3: 1617–1628.
Savickiene J, Treigyte G, Vistartaite G, Tunaitis V, Magnusson KE, Navakauskiene R . C/EBPalpha and PU.1 are involved in distinct differentiation responses of acute promyelocytic leukemia HL-60 and NB4 cells via chromatin remodeling. Differentiation 2011; 81: 57–67.
Brugnoli F, Lambertini E, Varin-Blank N, Piva R, Marchisio M, Grassilli S et al. Vav1 and PU.1 are recruited to the CD11b promoter in APL-derived promyelocytes: role of Vav1 in modulating PU.1-containing complexes during ATRA-induced differentiation. Exp Cell Res 2010; 316: 38–47.
Krysinska H, Hoogenkamp M, Ingram R, Wilson N, Tagoh H, Laslo P et al. A two-step, PU.1-dependent mechanism for developmentally regulated chromatin remodeling and transcription of the c-fms gene. Mol Cell Biol 2007; 27: 878–887.
Morgan GJ, Walker BA, Davies FE . The genetic architecture of multiple myeloma. Nat Rev Cancer 2012; 12: 335–348.
Ito T, Ando H, Suzuki T, Ogura T, Hotta K, Imamura Y et al. Identification of a primary target of thalidomide teratogenicity. Science 2010; 327: 1345–1350.
Lopez-Girona A, Heintel D, Zhang LH, Mendy D, Gaidarova S, Brady H et al. Lenalidomide downregulates the cell survival factor, interferon regulatory factor-4, providing a potential mechanistic link for predicting response. Br J Haematol 2011; 154: 325–336.
Endo S, Amano M, Nishimura N, Ueno N, Ueno S, Yuki H et al. Immunomodulatory drugs act as inhibitors of DNA methyltransferases and induce PU.1 up-regulation in myeloma cells. Biochem Biophys Res Commun 2015; 469: 236–242.
Miyoshi H, Takahashi M, Gage FH, Verma IM . Stable and efficient gene transfer into the retina using an HIV-based lentiviral vector. Proc Natl Acad Sci USA 1997; 94: 10319–10323.
Miyoshi H, Blomer U, Takahashi M, Gage FH, Verma IM . Development of a self-inactivating lentivirus vector. J Virol 1998; 72: 8150–8157.
Dolbeare F, Gratzner H, Pallavicini MG, Gray JW . Flow cytometric measurement of total DNA content and incorporated bromodeoxyuridine. Proc Natl Acad Sci USA 1983; 80: 5573–5577.
Lehtonen A, Veckman V, Nikula T, Lahesmaa R, Kinnunen L, Matikainen S et al. Differential expression of IFN regulatory factor 4 gene in human monocyte-derived dendritic cells and macrophages. J Immunol 2005; 175: 6570–6579.
Ohguchi H, Hideshima T, Bhasin MK, Gorgun GT, Santo L, Cea M et al. The KDM3A-KLF2-IRF4 axis maintains myeloma cell survival. Nat Commun 2016; 7: 10258.
Acknowledgements
This study was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (JSPS KAKENHI grant number 26461427).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Ueno, N., Nishimura, N., Ueno, S. et al. PU.1 acts as tumor suppressor for myeloma cells through direct transcriptional repression of IRF4. Oncogene 36, 4481–4497 (2017). https://doi.org/10.1038/onc.2017.79
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2017.79
This article is cited by
-
SUMOylation inhibition enhances multiple myeloma sensitivity to lenalidomide
Cancer Gene Therapy (2023)
-
PU.1 negatively regulates tumorigenesis in non-small-cell lung cancer
Medical Oncology (2023)
-
The complex relationship between MITF and the immune system: a Melanoma ImmunoTherapy (response) Factor?
Molecular Cancer (2020)