Abstract
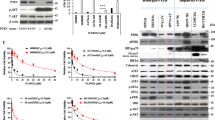
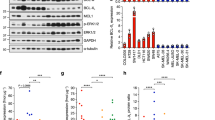
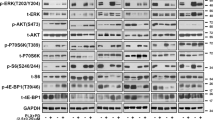
The melanoma incidence continues to increase, and the disease remains incurable for many due to its metastatic nature and high rate of therapeutic resistance. In particular, melanomas harboring BRAFV600E and PTEN mutations often are resistant to current therapies, including BRAF inhibitors (BRAFi) and immune checkpoint inhibitors. Abl kinases (Abl/Arg) are activated in melanomas and drive progression; however, their mechanism of activation has not been established. Here we elucidate a novel link between BRAFV600E/ERK signaling and Abl kinases. We demonstrate that BRAFV600E/ERK play a critical role in binding, phosphorylating and regulating Abl localization and Abl/Arg activation by Src family kinases. Importantly, Abl/Arg activation downstream of BRAFV600E has functional and biological significance, driving proliferation, invasion, as well as switch in epithelial–mesenchymal–transition transcription factor expression, which is known to be critical for melanoma cells to shift between differentiated and invasive states. Finally, we describe findings of high translational significance by demonstrating that Abl/Arg cooperate with PI3K/Akt/PTEN, a parallel pathway that is associated with intrinsic resistance to BRAFi and immunotherapy, as Abl/Arg and Akt inhibitors cooperate to prevent viability, cell cycle progression and in vivo growth of melanomas harboring mutant BRAF/PTEN. Thus, these data not only provide mechanistic insight into Abl/Arg regulation during melanoma development, but also pave the way for the development of new strategies for treating patients with melanomas harboring mutant BRAF/PTEN, which often are refractory to current therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Queirolo P, Picasso V, Spagnolo F . Combined BRAF and MEK inhibition for the treatment of BRAF-mutated metastatic melanoma. Cancer Treat Rev 2015; 41: 519–526.
Wilden SM, Lang BM, Mohr P, Grabbe S . Immune checkpoint inhibitors: a milestone in the treatment of melanoma. J Dtsch Dermatol Ges 2016; 14: 685–695.
Girotti MR, Saturno G, Lorigan P, Marais R . No longer an untreatable disease: how targeted and immunotherapies have changed the management of melanoma patients. Mol Oncol 2014; 8: 1140–1158.
Menzies AM, Haydu LE, Visintin L, Carlino MS, Howle JR, Thompson JF et al. Distinguishing clinicopathologic features of patients with V600E and V600K BRAF-mutant metastatic melanoma. Clin Cancer Res 2012; 18: 3242–3249.
Anderson WF, Pfeiffer RM, Tucker MA, Rosenberg PS . Divergent cancer pathways for early-onset and late-onset cutaneous malignant melanoma. Cancer 2009; 115: 4176–4185.
Maldonado JL, Fridlyand J, Patel H, Jain AN, Busam K, Kageshita T et al. Determinants of BRAF mutations in primary melanomas. J Natl Cancer Inst 2003; 95: 1878–1890.
Poynter JN, Elder JT, Fullen DR, Nair RP, Soengas MS, Johnson TM et al. BRAF and NRAS mutations in melanoma and melanocytic nevi. Melanoma Res 2006; 16: 267–273.
Cheung M, Sharma A, Madhunapantula SV, Robertson GP . Akt3 and mutant V600E B-Raf cooperate to promote early melanoma development. Cancer Res 2008; 68: 3429–3439.
Dankort D, Curley DP, Cartlidge RA, Nelson B, Karnezis AN, Damsky WE Jr et al. Braf(V600E) cooperates with Pten loss to induce metastatic melanoma. Nat Genet 2009; 41: 544–552.
Wheler J, Yelensky R, Falchook G, Kim KB, Hwu P, Tsimberidou AM et al. Next generation sequencing of exceptional responders with BRAF-mutant melanoma: implications for sensitivity and resistance. BMC Cancer 2015; 15: 61.
Paraiso KH, Xiang Y, Rebecca VW, Abel EV, Chen YA, Munko AC et al. PTEN loss confers BRAF inhibitor resistance to melanoma cells through the suppression of BIM expression. Cancer Res 2011; 71: 2750–2760.
Nathanson KL, Martin AM, Wubbenhorst B, Greshock J, Letrero R, D'Andrea K et al. Tumor genetic analyses of patients with metastatic melanoma treated with the BRAF inhibitor dabrafenib (GSK2118436). Clin Cancer Res 2013; 19: 4868–4878.
Peng W, Chen JQ, Liu C, Malu S, Creasy C, Tetzlaff MT et al. Loss of PTEN promotes resistance to T cell-mediated immunotherapy. Cancer Discov 2016; 6: 202–216.
Aguissa-Toure AH, Li G . Genetic alterations of PTEN in human melanoma. Cell Mol Life Sci 2012; 69: 1475–1491.
Xing F, Persaud Y, Pratilas CA, Taylor BS, Janakiraman M, She QB et al. Concurrent loss of the PTEN and RB1 tumor suppressors attenuates RAF dependence in melanomas harboring (V600E)BRAF. Oncogene 2012; 31: 446–457.
Vandamme N, Berx G . Melanoma cells revive an embryonic transcriptional network to dictate phenotypic heterogeneity. Front Oncol 2014; 4: 352.
Caramel J, Papadogeorgakis E, Hill L, Browne GJ, Richard G, Wierinckx A et al. A switch in the expression of embryonic EMT-inducers drives the development of malignant melanoma. Cancer Cell 2013; 24: 466–480.
Qiang L, He YY . Autophagy deficiency stabilizes TWIST1 to promote epithelial-mesenchymal transition. Autophagy 2014; 10: 1864–1865.
Denecker G, Vandamme N, Akay O, Koludrovic D, Taminau J, Lemeire K et al. Identification of a ZEB2-MITF-ZEB1 transcriptional network that controls melanogenesis and melanoma progression. Cell Death Differ 2014; 21: 1250–1261.
Weiss MB, Abel EV, Dadpey N, Aplin AE . FOXD3 modulates migration through direct transcriptional repression of TWIST1 in melanoma. Mol Cancer Res 2014; 12: 1314–1323.
Greuber EK, Smith-Pearson P, Wang J, Pendergast AM . Role of ABL family kinases in cancer: from leukaemia to solid tumours. Nat Rev Cancer 2013; 13: 559–571.
Ganguly SS, Plattner R . Activation of Abl family kinases in solid tumors. Genes Cancer 2012; 3: 414–425.
Wang J, Pendergast AM . The emerging role of ABL kinases in solid tumors. Trends Cancer 2015; 1: 110–123.
Ganguly SS, Fiore LS, Sims JT, Friend JW, Srinivasan D, Thacker MA et al. c-Abl and Arg are activated in human primary melanomas, promote melanoma cell invasion via distinct pathways, and drive metastatic progression. Oncogene 2012; 31: 1804–1816.
Fiore LS, Ganguly S, Sledziona J, Cibull ML, Wang C, Richards DL et al. c-Abl and Arg induce cathepsin-mediated lysosomal degradation of the NM23-H1 metastasis suppressor in invasive cancer. Oncogene 2014; 33: 4508–4520.
Zhang C, Yang C, Wang R, Jiao Y, Ampah KK, Wang X et al. c-Abl kinase is a regulator of alpha-v beta3 integrin mediated melanoma A375 cell migration. PLoS One 2013; 8: e66108.
Smith-Pearson PS, Greuber EK, Yogalingam G, Pendergast AM . Abl kinases are required for invadopodia formation and chemokine-induced invasion. J Biol Chem 2010; 285: 40201–40211.
Redondo P, Lloret P, Andreu EJ, Inoges S . Imatinib mesylate in cutaneous melanoma. J Invest Dermatol 2004; 123: 1208–1209.
Li R, Pendergast AM . Arg kinase regulates epithelial cell polarity by targeting beta1-integrin and small GTPase pathways. Curr Biol 2011; 21: 1534–1542.
Choi Y, Seeliger MA, Panjarian SB, Kim H, Deng X, Sim T et al. N-myristoylated c-Abl tyrosine kinase localizes to the endoplasmic reticulum upon binding to an allosteric inhibitor. J Biol Chem 2009; 284: 29005–29014.
Murray JC, Aldeghaither D, Wang S, Nasto RE, Jablonski SA, Tang Y et al. c-Abl modulates tumor cell sensitivity to antibody-dependent cellular cytotoxicity. Cancer Immunol Res 2014; 2: 1186–1198.
Tsai J, Lee JT, Wang W, Zhang J, Cho H, Mamo S et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc Natl Acad Sci USA 2008; 105: 3041–3046.
King AJ, Patrick DR, Batorsky RS, Ho ML, Do HT, Zhang SY et al. Demonstration of a genetic therapeutic index for tumors expressing oncogenic BRAF by the kinase inhibitor SB-590885. Cancer Res 2006; 66: 11100–11105.
Taagepera S, McDonald D, Loeb JE, Whitaker LL, McElroy AK, Wang JYJ et al. Nuclear-cytoplasmic shuttling of c-Abl tyrosine kinase. Proc Natl Acad Sci USA 1998; 95: 7457–7462.
Srinivasan D, Plattner R . Activation of Abl tyrosine kinases promotes invasion of aggressive breast cancer cells. Cancer Res 2006; 66: 5648–5655.
Plattner R, Kadlec L, DeMali KA, Kazlauskas A, Pendergast AM . c-Abl is activated by growth factors and Src family kinases and has a role in the cellular response to PDGF. Genes Dev 1999; 13: 2400–2411.
Riker AI, Enkemann SA, Fodstad O, Liu S, Ren S, Morris C et al. The gene expression profiles of primary and metastatic melanoma yields a transition point of tumor progression and metastasis. BMC Med Genomics 2008; 1: 13.
Barila D, Superti-Furga G . An intramolecular SH3-domain interaction regulates c-Abl activity. Nat Genet 1998; 18: 280–282.
Zhao F, He X, Wang Y, Shi F, Wu D, Pan M et al. Decrease of ZEB1 expression inhibits the B16F10 cancer stem-like properties. Biosci Trends 2015; 9: 325–334.
Sims JT, Ganguly SS, Bennett H, Friend WJ, Jessica T, Plattner R . Imatinib reverses doxorubicin resistance by affecting activation of STAT3-dependent NF-kB and HSP27/p38/AKT pathways and by inhibiting ABCB1. PLoS One 2013; 8: e55509.
Alexandropoulos K, Cheng G, Baltimore D . Proline-rich sequences that bind to Src homology 3 domains with individual specificities. Proc Natl Acad Sci USA 1995; 92: 3110–3114.
Richard G, Dalle S, Monet MA, Ligier M, Boespflug A, Pommier RM et al. ZEB1-mediated melanoma cell plasticity enhances resistance to MAPK inhibitors. EMBO Mol Med 2016; 8: 1143–1161.
Sini P, Cannas A, Koleske AJ, Di Fiore PP, Scita G . Abl-dependent tyrosine phosphorylation of Sos-1 mediates growth-factor-induced Rac activation. Nat Cell Biol 2004; 6: 268–274.
Srinivasan D, Kaetzel DM, Plattner R . Reciprocal regulation of Abl and receptor tyrosine kinases. Cell Signal 2009; 21: 1143–1150.
Lito P, Rosen N, Solit DB . Tumor adaptation and resistance to RAF inhibitors. Nat Med 2013; 19: 1401–1409.
Vultur A, Villanueva J, Krepler C, Rajan G, Chen Q, Xiao M et al. MEK inhibition affects STAT3 signaling and invasion in human melanoma cell lines. Oncogene 2014; 33: 1850–1861.
Girotti MR, Lopes F, Preece N, Niculescu-Duvaz D, Zambon A, Davies L et al. Paradox-breaking RAF inhibitors that also target SRC are effective in drug-resistant BRAF mutant melanoma. Cancer Cell 2015; 27: 85–96.
Tran A, Tawbi HA . A potential role for nilotinib in KIT-mutated melanoma. Expert Opin Investig Drugs 2012; 21: 861–869.
Karimkhani C, Gonzalez R, Dellavalle RP . A review of novel therapies for melanoma. Am J Clin Dermatol 2014; 15: 323–337.
Plattner R, Koleske AJ, Kazlauskas A, Pendergast AM . Bidirectional signaling links the abelson kinases to the platelet-derived growth factor receptor. Mol Cell Biol 2004; 24: 2573–2583.
Mitra S, Beach C, Feng GS, Plattner R . SHP-2 is a novel target of Abl kinases during cell proliferation. J Cell Sci 2008; 121: 3335–3346.
Li B, Cong F, Tan CP, Wang SX, Goff SP . Aph2, a protein with a zf-DHHC motif, interacts with c-Abl and has pro-apoptotic activity. J Biol Chem 2002; 277: 28870–28876.
Bondzi C, Grant S, Krystal GW . A novel assay for the measurement of Raf-1 kinase activity. Oncogene 2000; 19: 5030–5033.
Li X, Huang Y, Jiang J, Frank SJ . ERK-dependent threonine phosphorylation of EGF receptor modulates receptor downregulation and signaling. Cell Signal 2008; 20: 2145–2155.
Acknowledgements
This work was supported by the Markey Cancer Center Flow Cytometry, Biospecimen and Tissue Procurement, and Biostatistics Shared Resources and the Research Communication Office (P30CA177558). We thank Drs D’Orazio and Kyprianou for critically reading the manuscript. This work was supported by NIH Grant R01CA166499 to RP.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
This work was supported by an NIH/NCI grant to RP. CW is funded by NIH/NCI, but this work was not supported by his grants. The remaining authors declare no potential conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Rights and permissions
About this article
Cite this article
Jain, A., Tripathi, R., Turpin, C. et al. Abl kinase regulation by BRAF/ERK and cooperation with Akt in melanoma. Oncogene 36, 4585–4596 (2017). https://doi.org/10.1038/onc.2017.76
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2017.76
This article is cited by
-
MEK1/2 regulate normal BCR and ABL1 tumor-suppressor functions to dictate ATO response in TKI-resistant Ph+ leukemia
Leukemia (2023)
-
Oncogenic BRAF noncanonically promotes tumor metastasis by mediating VASP phosphorylation and filopodia formation
Oncogene (2023)
-
Role of the ABL tyrosine kinases in the epithelial–mesenchymal transition and the metastatic cascade
Cell Communication and Signaling (2021)
-
Combating acquired resistance to MAPK inhibitors in melanoma by targeting Abl1/2-mediated reactivation of MEK/ERK/MYC signaling
Nature Communications (2020)
-
Development of a yeast-based system to identify new hBRAFV600E functional interactors
Oncogene (2019)