Abstract
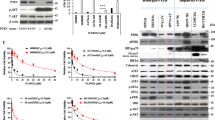
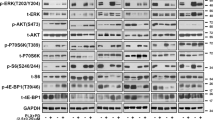
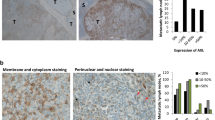
Malignant melanoma reveals rapidly increasing incidence and mortality rates worldwide. By now, BRAF inhibition is the standard therapy for advanced melanoma in patients carrying BRAF mutations. However, only approximately 50% of melanoma patients harbor therapeutically attackable BRAF mutations, and overall survival after treatment with BRAF inhibitors is modest. KRAS (Kirsten Rat sarcoma) proteins are acting upstream of BRAF and have a major role in human cancer. Recent approaches awaken the hope to use KRAS inhibition (KRASi) as a clinical tool. In this study, we identified wild-type KRAS as a novel therapeutic target in melanoma. KRASi functions synergistically with BRAF inhibition to reduce melanoma proliferation and to induce apoptosis independently of BRAF mutational status. Moreover, acquired resistance to BRAF inhibitors in melanoma is dependent on dynamic regulation of KRAS expression with subsequent AKT and extracellular-signal regulated kinase activation and can be overcome by KRASi. This suggests KRASi as novel approach in melanoma—alone or in combination with other therapeutic regimes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout









Similar content being viewed by others
Abbreviations
- AKT:
-
v-akt murine thymoma viral oncogene
- BRAF:
-
v-Raf murine sarcoma viral oncogene homolog B
- BRAFi:
-
BRAF inhibition/inhibitor
- CTR:
-
control
- DR:
-
Deltarasin
- ERK:
-
extracellular-signal regulated kinase
- KR:
-
KRAS (Kirsten Rat sarcoma)
- KRASi:
-
KRAS inhibition/inhibitor
- MAPK:
-
mitogen-activated protein kinase
- MEK:
-
mitogen-activated protein kinase kinase
- MEKi:
-
MEK inhibition/inhibitor
- NRAS:
-
neuroblastoma RAS viral oncogene homolog
- PI3K:
-
phosphatidylinositol-4,5-bisphosphate 3-kinase
- PLX:
-
PLX-4032 (Vemurafenib).
References
Leiter U, Eigentler T, Garbe C . Epidemiology of skin cancer. Adv Exp Med Biol 2014; 810: 120–140.
Harries M, Malvehy J, Lebbe C, Heron L, Amelio J, Szabo Z et al. Treatment patterns of advanced malignant melanoma (stage III-IV) - a review of current standards in Europe. Eur J Cancer 2016; 60: 179–189.
Dummer R, Hauschild A, Guggenheim M, Keilholz U, Pentheroudakis G . Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012; 23 (Suppl 7): vii86–vii91.
Chapuis AG, Lee SM, Thompson JA, Roberts IM, Margolin KA, Bhatia S et al. Combined IL-21-primed polyclonal CTL plus CTLA4 blockade controls refractory metastatic melanoma in a patient. J Exp Med 2016; 213: 1133–1139.
Matin RN, Chikh A, Chong SL, Mesher D, Graf M, Sanza P et al. p63 is an alternative p53 repressor in melanoma that confers chemoresistance and a poor prognosis. J Exp Med 2013; 210: 581–603.
Karimkhani C, Gonzalez R, Dellavalle RP . A review of novel therapies for melanoma. Am J Clin Dermatol 2014; 15: 323–337.
Luke JJ, Ott PA . New developments in the treatment of metastatic melanoma - role of dabrafenib-trametinib combination therapy. Drug Healthc Patient Saf 2014; 6: 77–88.
Monsma DJ, Cherba DM, Eugster EE, Dylewski DL, Davidson PT, Peterson CA et al. Melanoma patient derived xenografts acquire distinct Vemurafenib resistance mechanisms. Am J Cancer Res 2015; 5: 1507–1518.
Lito P, Rosen N, Solit DB . Tumor adaptation and resistance to RAF inhibitors. Nat Med 2013; 19: 1401–1409.
Nazarian R, Shi H, Wang Q, Kong X, Koya RC, Lee H et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature 2010; 468: 973–977.
Maertens O, Johnson B, Hollstein P, Frederick DT, Cooper ZA, Messiaen L et al. Elucidating distinct roles for NF1 in melanomagenesis. Cancer Discov 2013; 3: 338–349.
McCormick F . KRAS as a therapeutic target. Clin Cancer Res 2015; 21: 1797–1801.
Cox AD, Der CJ, Philips MR . Targeting RAS membrane association: back to the future for anti-RAS drug discovery? Clin Cancer Res 2015; 21: 1819–1827.
Stephen AG, Esposito D, Bagni RK, McCormick F . Dragging Ras back in the ring. Cancer Cell 2014; 25: 272–281.
Schmick M, Vartak N, Papke B, Kovacevic M, Truxius DC, Rossmannek L et al. KRas localizes to the plasma membrane by spatial cycles of solubilization, trapping and vesicular transport. Cell 2014; 157: 459–471.
Zimmermann G, Papke B, Ismail S, Vartak N, Chandra A, Hoffmann M et al. Small molecule inhibition of the KRAS-PDEdelta interaction impairs oncogenic KRAS signalling. Nature 2013; 497: 638–642.
Yuan TL, Fellmann C, Lee CS, Ritchie CD, Thapar V, Lee LC et al. Development of siRNA payloads to target KRAS-mutant cancer. Cancer Discov 2014; 4: 1182–1197.
Xue W, Dahlman JE, Tammela T, Khan OF, Sood S, Dave A et al. Small RNA combination therapy for lung cancer. Proc Natl Acad Sci USA 2014; 111: E3553–E3561.
Posch C, Cholewa BD, Vujic I, Sanlorenzo M, Ma J, Kim ST et al. Combined inhibition of MEK and Plk1 has synergistic antitumor activity in NRAS mutant melanoma. J Invest Dermatol 2015; 135: 2475–2483.
Milagre C, Dhomen N, Geyer FC, Hayward R, Lambros M, Reis-Filho JS et al. A mouse model of melanoma driven by oncogenic KRAS. Cancer Res 2010; 70: 5549–5557.
Gimotty PA, Van Belle P, Elder DE, Murry T, Montone KT, Xu X et al. Biologic and prognostic significance of dermal Ki67 expression, mitoses, and tumorigenicity in thin invasive cutaneous melanoma. J Clin Oncol 2005; 23: 8048–8056.
Depasquale I, Thompson WD . Microvessel density for melanoma prognosis. Histopathology 2005; 47: 186–194.
Villanueva J, Vultur A, Lee JT, Somasundaram R, Fukunaga-Kalabis M, Cipolla AK et al. Acquired resistance to BRAF inhibitors mediated by a RAF kinase switch in melanoma can be overcome by cotargeting MEK and IGF-1R/PI3K. Cancer Cell 2010; 18: 683–695.
Villanueva J, Infante JR, Krepler C, Reyes-Uribe P, Samanta M, Chen HY et al. Concurrent MEK2 mutation and BRAF amplification confer resistance to BRAF and MEK inhibitors in melanoma. Cell Rep 2013; 4: 1090–1099.
Johnson DB, Sosman JA . Therapeutic advances and treatment options in metastatic melanoma. JAMA Oncol 2015; 1: 380–386.
Burotto M, Chiou VL, Lee JM, Kohn EC . The MAPK pathway across different malignancies: a new perspective. Cancer 2014; 120: 3446–3456.
Sondergaard JN, Nazarian R, Wang Q, Guo D, Hsueh T, Mok S et al. Differential sensitivity of melanoma cell lines with BRAFV600E mutation to the specific Raf inhibitor PLX4032. J Transl Med 2010; 8: 39.
Wang J, Huang SK, Marzese DM, Hsu SC, Kawas NP, Chong KK et al. Epigenetic changes of EGFR have an important role in BRAF inhibitor-resistant cutaneous melanomas. J Invest Dermatol 2015; 135: 532–541.
Gross A, Niemetz-Rahn A, Nonnenmacher A, Tucholski J, Keilholz U, Fusi A . Expression and activity of EGFR in human cutaneous melanoma cell lines and influence of vemurafenib on the EGFR pathway. Target Oncol 2015; 10: 77–84.
Sale MJ, Cook SJ . That which does not kill me makes me stronger; combining ERK1/2 pathway inhibitors and BH3 mimetics to kill tumour cells and prevent acquired resistance. Br J Pharmacol 2013; 169: 1708–1722.
Wilson BE, Mochon E, Boxer LM . Induction of bcl-2 expression by phosphorylated CREB proteins during B-cell activation and rescue from apoptosis. Mol Cell Biol 1996; 16: 5546–5556.
Boucher MJ, Morisset J, Vachon PH, Reed JC, Laine J, Rivard N . MEK/ERK signaling pathway regulates the expression of Bcl-2, Bcl-X(L), and Mcl-1 and promotes survival of human pancreatic cancer cells. J Cell Biochem 2000; 79: 355–369.
Yang J, Song Q, Cai Y, Wang P, Wang M, Zhang D . RLIP76-dependent suppression of PI3K/AKT/Bcl-2 pathway by miR-101 induces apoptosis in prostate cancer. Biochem Biophys Res Commun 2015; 463: 900–906.
Grabocka E, Pylayeva-Gupta Y, Jones MJ, Lubkov V, Yemanaberhan E, Taylor L et al. Wild-type H- and N-Ras promote mutant K-Ras-driven tumorigenesis by modulating the DNA damage response. Cancer Cell 2014; 25: 243–256.
Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D . RAS oncogenes: weaving a tumorigenic web. Nat Rev Cancer 2011; 11: 761–774.
Chen Z, Cheng K, Walton Z, Wang Y, Ebi H, Shimamura T et al. A murine lung cancer co-clinical trial identifies genetic modifiers of therapeutic response. Nature 2012; 483: 613–617.
Jeng HH, Taylor LJ, Bar-Sagi D . Sos-mediated cross-activation of wild-type Ras by oncogenic Ras is essential for tumorigenesis. Nat Commun 2012; 3: 1168.
Ruiz C, Li J, Luttgen MS, Kolatkar A, Kendall JT, Flores E et al. Limited genomic heterogeneity of circulating melanoma cells in advanced stage patients. Phys Biol 2015; 12: 016008.
Wilson MA, Zhao F, Khare S, D'Andrea K, Wubbenhorst B, Roszik J et al. Copy number changes are associated with response to treatment with carboplatin, paclitaxel, and sorafenib in melanoma. Clin Cancer Res 2015; 22: 374–382.
Sweetlove M, Wrightson E, Kolekar S, Rewcastle GW, Baguley BC, Shepherd PR et al. Inhibitors of pan-PI3K signaling synergize with BRAF or MEK inhibitors to prevent BRAF-mutant melanoma cell growth. Front Oncol 2015; 5: 135.
Lavoie H, Therrien M . Regulation of RAF protein kinases in ERK signalling. Nat Rev Mol Cell Biol 2015; 16: 281–298.
Herrero A, Pinto A, Colon-Bolea P, Casar B, Jones M, Agudo-Ibanez L et al. Small molecule inhibition of ERK dimerization prevents tumorigenesis by RAS-ERK pathway oncogenes. Cancer Cell 2015; 28: 170–182.
Richman J, Martin-Liberal J, Diem S, Larkin J . BRAF and MEK inhibition for the treatment of advanced BRAF mutant melanoma. Expert Opin Pharmacother 2015; 16: 1285–1297.
Queirolo P, Picasso V, Spagnolo F . Combined BRAF and MEK inhibition for the treatment of BRAF-mutated metastatic melanoma. Cancer Treat Rev 2015; 41: 519–526.
Whittaker SR, Cowley GS, Wagner S, Luo F, Root DE, Garraway LA . Combined pan-RAF and MEK inhibition overcomes multiple resistance mechanisms to selective RAF inhibitors. Mol Cancer Ther 2015; 14: 2700–2711.
Perna D, Karreth FA, Rust AG, Perez-Mancera PA, Rashid M, Iorio F et al. BRAF inhibitor resistance mediated by the AKT pathway in an oncogenic BRAF mouse melanoma model. Proc Natl Acad Sci USA 2015; 112: E536–E545.
Shi H, Hugo W, Kong X, Hong A, Koya RC, Moriceau G et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov 2014; 4: 80–93.
Hugo W, Shi H, Sun L, Piva M, Song C, Kong X et al. Non-genomic and immune evolution of melanoma acquiring MAPKi resistance. Cell 2015; 162: 1271–1285.
Smyth T, Paraiso KH, Hearn K, Rodriguez-Lopez AM, Munck JM, Haarberg HE et al. Inhibition of HSP90 by AT13387 delays the emergence of resistance to BRAF inhibitors and overcomes resistance to dual BRAF and MEK inhibition in melanoma models. Mol Cancer Ther 2014; 13: 2793–2804.
Carlino MS, Kwan V, Miller DK, Saunders CA, Yip D, Nagrial AM et al. New RAS-mutant pancreatic adenocarcinoma with combined BRAF and MEK inhibition for metastatic melanoma. J Clin Oncol 2015; 33: e52–e56.
The KRAS-PDEdelta interaction is a therapeutic target. Cancer Discov 2013; 3: OF20.
Milroy LG, Ottmann C . The renaissance of Ras. ACS Chem Biol 2014; 9: 2447–2458.
Ledford H . Cancer: the Ras renaissance. Nature 2015; 520: 278–280.
Loi S, Dushyanthen S, Beavis PA, Salgado R, Denkert C, Savas P et al. RAS/MAPK activation is associated with reduced tumor-infiltrating lymphocytes in triple-negative breast cancer: therapeutic cooperation between MEK and PD-1/PD-L1 immune checkpoint inhibitors. Clin Cancer Res 2015; 22: 1499–1509.
Fan X, Quezada SA, Sepulveda MA, Sharma P, Allison JP . Engagement of the ICOS pathway markedly enhances efficacy of CTLA-4 blockade in cancer immunotherapy. J Exp Med 2014; 211: 715–725.
Acknowledgements
This work was supported by the German Research Association (DFG) (Research Training Group ‘RTG 1962/1’, University of Erlangen), the German Cancer Aid (Deutsche Krebshilfe), the Bavarian Research Network for Molecular Biosystems (BioSysNet) and the Interdisciplinary Center for Clinical Research (IZKF) Erlangen (J55, to PD and D24, to AB). We thank Meenhard Herlyn (The Wistar Institute, Philadelphia, USA) for providing the resistant cell lines. Furthermore, we thank Rudolf Jung, Annette Serwotka and Darleen Schönwälder for technical assistance.
Author contributions
PD, CH and AKB conceived the project, analyzed the data and wrote the paper. PD designed and performed most of the experiments. TS helped in planning and performing mouse experiments. SK contributed to data analysis and manuscript creation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Dietrich, P., Kuphal, S., Spruss, T. et al. Wild-type KRAS is a novel therapeutic target for melanoma contributing to primary and acquired resistance to BRAF inhibition. Oncogene 37, 897–911 (2018). https://doi.org/10.1038/onc.2017.391
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2017.391
This article is cited by
-
C-Jun drives melanoma progression in PTEN wild type melanoma cells
Cell Death & Disease (2019)