Abstract
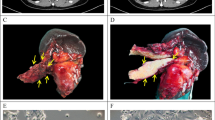
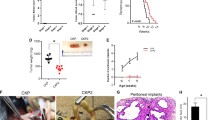
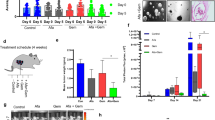
Our increasing knowledge of the mechanisms behind the progression of pancreatic cancer (PC) has not yet translated into effective treatments. Many promising drugs have failed in the clinic, highlighting the need for better preclinical models to assess drug efficacy and characterize mechanisms of resistance. Using different experimental models, including patient-derived xenografts (PDXs), we gauged the efficacy of therapies aimed at two hallmark lesions of PCs: activation of signaling pathways by oncogenic KRAS and inactivation of tumor-suppressor genes. Although the drug targeting inactivation of tumor suppressors by DNA methylation had little effect, the inhibition of Mek, a K-Ras effector, in combination with the standard of care (chemotherapy consisting of gemcitabine/Nab-paclitaxel), reduced the growth of three out of five PC-PDXs and impaired metastasis. The two least responding PC-PDXs were composed of genetically diverse cells, which displayed sensitivities to the Mek inhibitor differing by >10-fold. Unexpectedly, our analysis of this genetic diversity unveiled different KRAS mutations. As mutation in KRAS occurs early during progression, this heterogeneity may reflect the simultaneous appearance of different malignant cellular clones or, alternatively, that cells containing two mutations of KRAS are selected during tumor evolution. In vitro and in vivo analyses indicated that the intratumoral heterogeneity, along with the selective pressure imposed by the Mek inhibitor, resulted in rapid selection of resistant cells. Together with the gemcitabine/Nab-paclitaxel backbone, Mek inhibition could be effective in treatment of PC. However, resistance because of intratumoral heterogeneity is likely to develop frequently, pointing to the necessity of identifying the factors and mechanisms of resistance to further develop this therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Ryan DP, Hong TS, Bardeesy N . Pancreatic adenocarcinoma. N Engl J Med 2014; 371: 1039–1049.
Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997; 15: 2403–2413.
Hoff, Von DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 2013; 369: 1691–1703.
Biankin AV, Waddell N, Kassahn KS, Gingras M-C, Muthuswamy LB, Johns AL et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature 2012; 491: 399–405.
Collins MA, Bednar F, Zhang Y, Brisset J-C, Galbán S, Galbán CJ et al. Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice. J Clin Invest 2012; 122: 639–653.
Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 2007; 445: 656–660.
Collisson EA, Trejo CL, Silva JM, Gu S, Korkola JE, Heiser LM et al. A central role for RAF->MEK->ERK signaling in the genesis of pancreatic ductal adenocarcinoma. Cancer Discov 2012; 2: 685–693.
Weisenberger DJ . Characterizing DNA methylation alterations from the Cancer Genome Atlas. J Clin Invest 2014; 124: 17–23.
Missiaglia E, Donadelli M, Palmieri M, Crnogorac-Jurcevic T, Scarpa A, Lemoine NR . Growth delay of human pancreatic cancer cells by methylase inhibitor 5-aza-2′-deoxycytidine treatment is associated with activation of the interferon signalling pathway. Oncogene 2005; 24: 199–211.
Kopetz S, Kopetz S, Lemos R, Lemos R, Powis G, Powis G . The promise of patient-derived xenografts: the best laid plans of mice and men. Clin Cancer Res 2012; 18: 5160–5162.
Kim MP, Evans DB, Wang H, Abbruzzese JL, Fleming JB, Gallick GE . Generation of orthotopic and heterotopic human pancreatic cancer xenografts in immunodeficient mice. Nat Protoc 2009; 4: 1670–1680.
Fisher R, Pusztai L, Swanton C . Cancer heterogeneity: implications for targeted therapeutics. Br J Cancer 2013; 108: 479–485.
Byrne AT, Alférez DG, Amant F, Annibali D, Arribas J, Biankin AV et al. Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nat Rev Cancer 2017; 17: 254–268.
Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 2009; 324: 1457–1461.
Gore J, Korc M . Pancreatic cancer stroma: friend or foe? Cancer Cell 2014; 25: 711–712.
Özdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu C-C, Simpson TR et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 2014; 25: 719–734.
Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA et al. Stromal elements Actto restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 2014; 25: 735–747.
Van Cutsem E, Hidalgo M, Bazin I, Canon J-L, Poddubskaya E, Manojlovic N et al. Phase II randomized trial of MEK inhibitor pimasertib or placebo combined with gemcitabine in the first-line treatment of metastatic pancreatic cancer. J Clin Oncol 2015; 33; (Suppl3)Abstract 344.
Borroto A, Ruiz-Paz S, la Torre de TV, Borrell-Pages M, Merlos-Suarez A, Pandiella A et al. Impaired trafficking and activation of tumor necrosis factor-alpha-converting enzyme in cell mutants defective in protein ectodomain shedding. J Biol Chem 2003; 278: 25933–25939.
Acknowledgements
We thank Faye Su from Novartis and Daniel Pierce from Celgene for providing reagents and valuable insights. This work was supported by funds from the Instituto de Salud Carlos III (PI16/00253 and CIBER-ONC). KP was supported by the postdoctoral program from the AECC. FB was supported by the AGAUR-FI DGR-2014 predoctoral fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Pedersen, K., Bilal, F., Bernadó Morales, C. et al. Pancreatic cancer heterogeneity and response to Mek inhibition. Oncogene 36, 5639–5647 (2017). https://doi.org/10.1038/onc.2017.174
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2017.174
This article is cited by
-
The beginning of the end for conventional RECIST — novel therapies require novel imaging approaches
Nature Reviews Clinical Oncology (2019)
-
Novel agents for pancreatic ductal adenocarcinoma: emerging therapeutics and future directions
Journal of Hematology & Oncology (2018)