Abstract
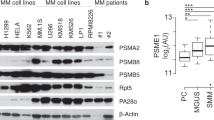
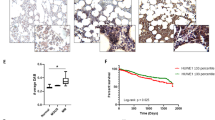
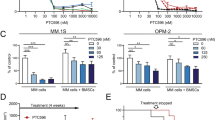
Proteasome inhibition is an effective therapy for multiple myeloma (MM) patients; however, the emergence of drug resistance is common. Novel therapeutic strategies to overcome proteasome inhibitor resistance are needed. In this study, we examined whether targeting deubiquitylating (DUB) enzymes upstream of 20S proteasome overcomes proteasome inhibitor resistance. Gene expression analysis, immunohistochemical studies of MM patient bone marrow, reverse transcription–PCR and protein analysis show that Rpn11/POH1, a DUB enzyme upstream of 20S proteasome, is more highly expressed in patient MM cells than in normal plasma cells. Importantly, Rpn11 expression directly correlates with poor patient survival. Loss-of-function studies show that Rpn11-siRNA knockdown decreases MM cell viability. Pharmacological inhibition of Rpn11 with O-phenanthroline (OPA) blocks cellular proteasome function, induces apoptosis in MM cells and overcomes resistance to proteasome inhibitor bortezomib. Mechanistically, Rpn11 inhibition in MM cells activates caspase cascade and endoplasmic stress response signaling. Human MM xenograft model studies demonstrate that OPA treatment reduces progression of tumor growth and prolongs survival in mice. Finally, blockade of Rpn11 increases the cytotoxic activity of anti-MM agents lenalidomide, pomalidomide or dexamethasone. Overall, our preclinical data provide the rationale for targeting DUB enzyme Rpn11 upstream of 20S proteasome to enhance cytotoxicity and overcome proteasome inhibitor resistance in MM.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Lee MJ, Lee BH, Hanna J, King RW, Finley D . Trimming of ubiquitin chains by proteasome-associated deubiquitinating enzymes. Mol Cell Proteomics 2011; 10: 003871.
Lander GC, Estrin E, Matyskiela ME, Bashore C, Nogales E, Martin A . Complete subunit architecture of the proteasome regulatory particle. Nature 2012; 482: 186–191.
Lasker K, Forster F, Bohn S, Walzthoeni T, Villa E, Unverdorben P et al. Molecular architecture of the 26S proteasome holocomplex determined by an integrative approach. Proc Natl Acad Sci USA 2012; 109: 1380–1387.
Goldberg AL . Protein degradation and protection against misfolded or damaged proteins. Nature 2003; 426: 895–899.
Pickart CM, Eddins MJ . Ubiquitin: structures, functions, mechanisms. Biochim Biophys Acta 2004; 1695: 55–72.
Adams J . The proteasome: a suitable antineoplastic target. Nat Rev Cancer 2004; 4: 349–360.
Chauhan D, Hideshima T, Anderson KC . Proteasome inhibition in multiple myeloma: therapeutic implication. Annu Rev Pharmacol Toxicol 2005; 45: 465–476.
Hershko A . The ubiquitin system for protein degradation and some of its roles in the control of the cell-division cycle (Nobel lecture). Angew Chem Int Ed Engl 2005; 44: 5932–5943.
Finley D . Recognition and processing of ubiquitin-protein conjugates by the proteasome. Annu Rev Biochem 2009; 78: 477–513.
Hussain S, Zhang Y, Galardy PJ . DUBs and cancer: the role of deubiquitinating enzymes as oncogenes, non-oncogenes and tumor suppressors. Cell Cycle 2009; 8: 1688–1697.
Chauhan D, Tian Z, Nicholson B, Kumar KG, Zhou B, Carrasco R et al. A small molecule inhibitor of ubiquitin-specific protease-7 induces apoptosis in multiple myeloma cells and overcomes bortezomib resistance. Cancer Cell 2012; 22: 345–358.
Lim KH, Baek KH . Deubiquitinating enzymes as therapeutic targets in cancer. Curr Pharm Des 2013; 19: 4039–4052.
Liu CW, Jacobson AD . Functions of the 19S complex in proteasomal degradation. Trends Biochem Sci 2013; 38: 103–110.
Bhattacharyya S, Yu H, Mim C, Matouschek A . Regulated protein turnover: snapshots of the proteasome in action. Nat Rev Mol Cell Biol 2014; 15: 122–133.
D'Arcy P, Brnjic S, Olofsson MH, Fryknas M, Lindsten K, De Cesare M et al. Inhibition of proteasome deubiquitinating activity as a new cancer therapy. Nat Med 2011; 17: 1636–1640.
Borodovsky A, Kessler BM, Casagrande R, Overkleeft HS, Wilkinson KD, Ploegh HL . A novel active site-directed probe specific for deubiquitylating enzymes reveals proteasome association of USP14. EMBO J 2001; 20: 5187–5196.
Tian Z, D'Arcy P, Wang X, Ray A, Tai YT, Hu Y et al. A novel small molecule inhibitor of deubiquitylating enzyme USP14 and UCHL5 induces apoptosis in multiple myeloma and overcomes bortezomib resistance. Blood 2014; 123: 706–716.
Yao T, Cohen RE . A cryptic protease couples deubiquitination and degradation by the proteasome. Nature 2002; 419: 403–407.
Verma R, Aravind L, Oania R, McDonald WH, Yates JR 3rd, Koonin EV et al. Role of Rpn11 metalloprotease in deubiquitination and degradation by the 26S proteasome. Science 2002; 298: 611–615.
Maytal-Kivity V, Reis N, Hofmann K, Glickman MH . MPN+, a putative catalytic motif found in a subset of MPN domain proteins from eukaryotes and prokaryotes, is critical for Rpn11 function. BMC Biochem 2002; 3: 28.
Koulich E, Li X, DeMartino GN . Relative structural and functional roles of multiple deubiquitylating proteins associated with mammalian 26S proteasome. Mol Biol Cell 2008; 19: 1072–1082.
Gallery M, Blank JL, Lin Y, Gutierrez JA, Pulido JC, Rappoli D et al. The JAMM motif of human deubiquitinase Poh1 is essential for cell viability. Mol Cancer Ther 2007; 6: 262–268.
Menendez-Benito V, Verhoef LG, Masucci MG, Dantuma NP . Endoplasmic reticulum stress compromises the ubiquitin-proteasome system. Hum Mol Genet 2005; 14: 2787–2799.
Chauhan D, Uchiyama H, Akbarali Y, Urashima M, Yamamoto K, Libermann TA et al. Multiple myeloma cell adhesion-induced interleukin-6 expression in bone marrow stromal cells involves activation of NF-kappa B. Blood 1996; 87: 1104–1112.
Ray A, Das DS, Song Y, Richardson P, Munshi NC, Chauhan D et al. Targeting PD1-PDL1 immune checkpoint in plasmacytoid dendritic cell interactions with T cells, natural killer cells and multiple myeloma cells. Leukemia 2015; 29: 1441–1444.
Bassal S, Nomura N, Venter D, Brand K, McKay MJ, van der Spek PJ . Characterization of a novel human cell-cycle-regulated homologue of Drosophila dlg1. Genomics 2001; 77: 5–7.
Watson RA, Rollason TP, Reynolds GM, Murray PG, Banks L, Roberts S . Changes in expression of the human homologue of the Drosophila discs large tumour suppressor protein in high-grade premalignant cervical neoplasias. Carcinogenesis 2002; 23: 1791–1796.
Chauhan D, Singh AV, Brahmandam M, Carrasco R, Bandi M, Hideshima T et al. Functional interaction of plasmacytoid dendritic cells with multiple myeloma cells: a therapeutic target. Cancer Cell 2009; 16: 309–323.
Song Y, Ray A, Li S, Das DS, Tai YT, Carrasco RD et al. Targeting proteasome ubiquitin receptor Rpn13 in multiple myeloma. Leukemia 2016; 30: 1877–1886.
Wang B, Ma A, Zhang L, Jin WL, Qian Y, Xu G et al. POH1 deubiquitylates and stabilizes E2F1 to promote tumour formation. Nat Commun 2015; 6: 8704.
Bergsagel PL, Kuehl WM . Molecular pathogenesis and a consequent classification of multiple myeloma. J Clin Oncol 2005; 23: 6333–6338.
Bergsagel PL, Chesi M, Nardini E, Brents LA, Kirby SL, Kuehl WM . Promiscuous translocations into immunoglobulin heavy chain switch regions in multiple myeloma. Proc Natl Acad Sci USA 1996; 93: 13931–13936.
Greenstein S, Krett NL, Kurosawa Y, Ma C, Chauhan D, Hideshima T et al. Characterization of the MM.1 human multiple myeloma (MM) cell lines: a model system to elucidate the characteristics, behavior, and signaling of steroid-sensitive and -resistant MM cells. Exp Hematol 2003; 31: 271–282.
Avet-Loiseau H, Attal M, Moreau P, Charbonnel C, Garban F, Hulin C et al. Genetic abnormalities and survival in multiple myeloma: the experience of the Intergroupe Francophone du Myelome. Blood 2007; 109: 3489–3495.
Burger R, Guenther A, Bakker F, Schmalzing M, Bernand S, Baum W et al. Gp130 and ras mediated signaling in human plasma cell line INA-6: a cytokine-regulated tumor model for plasmacytoma. Hematol J 2001; 2: 42–53.
Spataro V, Toda T, Craig R, Seeger M, Dubiel W, Harris AL et al. Resistance to diverse drugs and ultraviolet light conferred by overexpression of a novel human 26S proteasome subunit. J Biol Chem 1997; 272: 30470–30475.
Spataro V, Simmen K, Realini CA . The essential 26S proteasome subunit Rpn11 confers multidrug resistance to mammalian cells. Anticancer Res 2002; 22: 3905–3909.
Rinaldi T, Ricci C, Porro D, Bolotin-Fukuhara M, Frontali L . A mutation in a novel yeast proteasomal gene, RPN11/MPR1, produces a cell cycle arrest, overreplication of nuclear and mitochondrial DNA, and an altered mitochondrial morphology. Mol Biol Cell 1998; 9: 2917–2931.
Rinaldi T, Ricordy R, Bolotin-Fukuhara M, Frontali L . Mitochondrial effects of the pleiotropic proteasomal mutation mpr1/rpn11: uncoupling from cell cycle defects in extragenic revertants. Gene 2002; 286: 43–51.
Rinaldi T, Pick E, Gambadoro A, Zilli S, Maytal-Kivity V, Frontali L et al. Participation of the proteasomal lid subunit Rpn11 in mitochondrial morphology and function is mapped to a distinct C-terminal domain. Biochem J 2004; 381: 275–285.
Richardson PG, Xie W, Jagannath S, Jakubowiak A, Lonial S, Raje NS et al. A phase 2 trial of lenalidomide, bortezomib, and dexamethasone in patients with relapsed and relapsed/refractory myeloma. Blood 2014; 123: 1461–1469.
Richardson PG, Weller E, Lonial S, Jakubowiak AJ, Jagannath S, Raje NS et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood 2010; 116: 679–686.
Richardson PG, Weller E, Jagannath S, Avigan DE, Alsina M, Schlossman RL et al. Multicenter, phase I, dose-escalation trial of lenalidomide plus bortezomib for relapsed and relapsed/refractory multiple myeloma. J Clin Oncol 2009; 27: 5713–5719.
Richardson PG, Siegel DS, Vij R, Hofmeister CC, Baz R, Jagannath S et al. Pomalidomide alone or in combination with low-dose dexamethasone in relapsed and refractory multiple myeloma: a randomized phase 2 study. Blood 2014; 123: 1826–1832.
Kuhn DJ, Chen Q, Voorhees PM, Strader JS, Shenk KD, Sun CM et al. Potent activity of carfilzomib, a novel, irreversible inhibitor of the ubiquitin-proteasome pathway, against preclinical models of multiple myeloma. Blood 2007; 110: 3281–3290.
Chauhan D, Singh A, Brahmandam M, Podar K, Hideshima T, Richardson P et al. Combination of proteasome inhibitors bortezomib and NPI-0052 trigger in vivo synergistic cytotoxicity in multiple myeloma. Blood 2008; 111: 1654–1664.
Chauhan D, Ray A, Viktorsson K, Spira J, Paba-Prada C, Munshi N et al. In vitro and in vivo antitumor activity of a novel alkylating agent, melphalan-flufenamide, against multiple myeloma cells. Clin Cancer Res 2013; 19: 3019–3031.
Acknowledgements
This investigation was supported by National Institutes of Health Specialized Programs of Research Excellence (SPORE) Grants P50100707, R01CA207237 and RO1CA050947. KCA is an American Cancer Society Clinical Research Professor.
Author contributions
DC conceived the project, designed research, analyzed data and wrote the manuscript; YS designed and performed the experiments and interpreted data; SL helped in RT–PCR and animal studies; AR and DSD helped in cytotoxicity assays; JQ helped in western studies; MKS performed the GEP data set analyses; NM and Y-TT provided patient samples; RDC helped in immunohistochemistry analysis; and KCA reviewed the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
KCA is on advisory board of Celgene, Millenium, Gilead and Bristol Myers Squibb, and is a Scientific Founder of Acetylon, Oncopep and C4 Therapeutics. The other authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Song, Y., Li, S., Ray, A. et al. Blockade of deubiquitylating enzyme Rpn11 triggers apoptosis in multiple myeloma cells and overcomes bortezomib resistance. Oncogene 36, 5631–5638 (2017). https://doi.org/10.1038/onc.2017.172
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2017.172
This article is cited by
-
The function and mechanism of PSMD14 in promoting progression and resistance to anlotinib in osteosarcoma
Cancer Cell International (2023)
-
Protein degradation: expanding the toolbox to restrain cancer drug resistance
Journal of Hematology & Oncology (2023)
-
PSMD12 interacts with CDKN3 and facilitates pancreatic cancer progression
Cancer Gene Therapy (2023)
-
The prognostic role of PSMD14 in head and neck squamous cell carcinoma
Journal of Cancer Research and Clinical Oncology (2023)
-
The emerging role of deubiquitylating enzymes as therapeutic targets in cancer metabolism
Cancer Cell International (2022)