Abstract
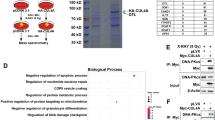
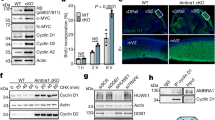
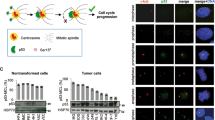
CUL9 is a member of the cullin family of E3 ubiquitin ligases, and it localizes predominantly in the cytoplasm. Deletion of Cul9 in mice results in increased DNA damage, widespread aneuploidy, spontaneous tumor development, accelerated Eμ-Myc-induced lymphomagenesis and susceptibility to carcinogenesis. CUL9 binds to p53 and causes cell apoptosis when ectopically expressed. Whether the function of CUL9 in maintaining genomic integrity and suppressing tumorigenesis is linked to p53 has not been genetically tested. Here we report that deletion of CUL9 in human cells results in attenuated p21 induction and impaired cellular response to DNA damage. We show that disruption of Cul9-p53 binding in mouse embryo fibroblasts (MEFs) by a knock-in mutation in Cul9 (Δp53) increases S-phase cell population, accumulates DNA damage during DNA replication and decreases apoptosis to both endogenous and exogenous DNA-damaging agents. The extent of these alterations in Cul9Δp53 MEFs is indistinguishable to those seen in Cul9−/− MEFs and comparable to those seen in p53−/− MEFs. Deletion of CUL9 in p53 null cells does not lead to further increase of DNA damages. Both Cul9−/− and Cul9Δp53 MEFs proliferate faster and undergo spontaneous immortalization while retaining both Arf and p53. These results demonstrate that the functions of CUL9 in regulating cell proliferation and maintaining genomic integrity are mainly mediated by p53, and that CUL9 is a critical p53 activator.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Lane D, Levine A . p53 Research: the past thirty years and the next thirty years. Cold Spring Harb Perspect Biol 2010; 2: a000893.
Donehower LA, Lozano G . 20 years studying p53 functions in genetically engineered mice. Nat Rev Cancer 2009; 9: 831–841.
Hollstein M, Sidransky D, Vogelstein B, Harris CC . p53 mutations in human cancers. Science 1991; 253: 49–53.
Stracquadanio G, Wang X, Wallace MD, Grawenda AM, Zhang P, Hewitt J et al. The importance of p53 pathway genetics in inherited and somatic cancer genomes. Nat Rev Cancer 2016; 16: 251–265.
Marin I . Diversification of the cullin family. BMC Evol Biol 2009; 9: 267.
Andrews P, He YJ, Xiong Y . Cytoplasmic localized ubiquitin ligase cullin 7 binds to p53 and promotes cell growth by antagonizing p53 function. Oncogene 2006; 25: 4534–4548.
Nikolaev AY, Li M, Puskas N, Qin J, Gu W . Parc: a cytoplasmic anchor for p53. Cell 2003; 112: 29–40.
Li Z, Pei XH, Yan J, Yan F, Cappell KM, Whitehurst AW et al. CUL9 mediates the functions of the 3M complex and ubiquitylates survivin to maintain genome integrity. Mol Cell 2014; 54: 805–819.
Yan J, Yan F, Li Z, Sinnott B, Cappell KM, Yu Y et al. The 3M complex maintains microtubule and genome integrity. Mol Cell 2014; 54: 791–804.
Vilenchik MM, Knudson AG . Endogenous DNA double-strand breaks: production, fidelity of repair, and induction of cancer. Proc Natl Acad Sci USA 2003; 100: 12871–12876.
Rogakou EP, Boon C, Redon C, Bonner WM . Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol 1999; 146: 905–916.
Pei XH, Bai F, Li Z, Smith MD, Whitewolf G, Jin R et al. Cytoplasmic CUL9/PARC ubiquitin ligase is a tumor suppressor and promotes p53-dependent apoptosis. Cancer Res 2011; 71: 2969–2977.
Salic A, Mitchison TJ . A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc Natl Acad Sci USA 2008; 105: 2415–2420.
Sharpless NE. Preparation and immortlization of primary murine cells. In: Celis JE (ed.). Cell Biology (Third Edition): A Laboratory Handbook, vol. 1, 2006, pp. 223–228.
Harvey DM, Levine AJ . p53 alteration is a common event in the spontaneous immortalization of primary BALB/c murine embryo fibroblasts. Genes Dev 1991; 5: 2375–2385.
Kamijo T, Zindy F, Roussel MF, Quelle DE, Downing JR, Ashmun RA et al. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell 1997; 91: 649–659.
Meek DW, Anderson CW . Posttranslational modification of p53: cooperative integrators of function. Cold Spring Harb Perspect Biol 2009; 1: a000950.
Montes de Oca Luna R, Wagner DS, Lozano G . Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature 1995; 378: 203–206.
Jones SN, Roe AE, Donehower LA, Bradley A . Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature 1995; 378: 206–208.
Parant J, Chavez-Reyes A, Little NA, Yan W, Reinke V, Jochemsen AG et al. Rescue of embryonic lethality in Mdm4-null mice by loss of Trp53 suggests a nonoverlapping pathway with MDM2 to regulate p53. Nat Genet 2001; 29: 92–95.
Pant V, Lozano G . Limiting the power of p53 through the ubiquitin proteasome pathway. Genes Dev 2014; 28: 1739–1751.
Acknowledgements
We thank Hui Xiao Chao, Jeremy Purvis and Matthew Smith for reading the manuscript, Dale Ramsden for discussion throughout this study, and members of the Xiong Laboratory for helpful discussions and encouragement. This work was supported by National Institutes of Health (grant R01CA068377 to YX) and a Team Innovation Award by the UNC Lineberger Comprehensive Cancer Center’s University Cancer Research Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Li, Z., Xiong, Y. Cytoplasmic E3 ubiquitin ligase CUL9 controls cell proliferation, senescence, apoptosis and genome integrity through p53. Oncogene 36, 5212–5218 (2017). https://doi.org/10.1038/onc.2017.141
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2017.141
This article is cited by
-
Noncanonical assembly, neddylation and chimeric cullin–RING/RBR ubiquitylation by the 1.8 MDa CUL9 E3 ligase complex
Nature Structural & Molecular Biology (2024)
-
CRL2APPBP2-mediated TSPYL2 degradation counteracts human mesenchymal stem cell senescence
Science China Life Sciences (2024)
-
miRNA profiling of primary lamb testicle cells infected with lumpy skin disease virus
Archives of Virology (2023)
-
CDK1–cyclin-B1-induced kindlin degradation drives focal adhesion disassembly at mitotic entry
Nature Cell Biology (2022)
-
Regulation of cell cycle drivers by Cullin-RING ubiquitin ligases
Experimental & Molecular Medicine (2020)