Abstract
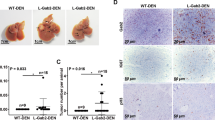
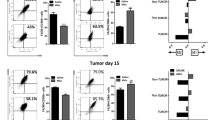
Hepatocellular carcinoma (HCC) is linked to inflammation and immunosuppression. Chemerin is highly expressed in the liver and implicated in the regulation of inflammation. However, the role of chemerin in HCC remains unclear. In this study, we aimed to investigate whether chemerin is able to influence HCC progression by regulating tumor-associated inflammation. Here we demonstrated that chemerin significantly decreased in blood and tumor tissues of HCC patients, and tumor chemerin levels were inversely associated with the prognosis. In an orthotopic mouse model of HCC, Rarres2−/− mice exhibited aggressive tumor growth and lung metastasis, whereas chemerin overexpression greatly inhibited tumor growth. The tumor-inhibitory effect of chemerin was accompanied by a shift in tumor-infiltrating immune cells from myeloid-derived suppressive cells (MDSCs) to interferon-γ+T cells and decreased tumor angiogenesis. Furthermore, we demonstrated that the tumor-inhibitory effect of chemerin was partly dependent on T cells, as chemerin overexpression could inhibit tumor growth, albeit to a lesser extent, in Rag1−/− mice when compared with wild-type controls. Mechanistically, chemerin inhibited nuclear factor-κB activation and the expression of granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-2 (IL-6) by tumor cells and tumor-associated endothelial cell, respectively, via its receptors, and consequently, MDSC induction was impaired, leading to restoration of antitumor T-cell response and decreased tumor angiogenesis. Clinically, systemic and tumor levels of chemerin were found to inversely correlate with circulating concentrations of GM-CSF or IL-6 and tumor-infiltrating myeloid cells, respectively, in HCC patients. Moreover, neutralization of GM-CSF and IL-6 abrogated HCC progression and MDSC accumulation in Rarres2−/− mice. In conclusion, our study reveals the tumor-inhibitory effect of chemerin by suppressing inflammatory tumor microenvironment with therapeutic implications for inflammation-associated cancer-like HCC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Makarova-Rusher OV, Medina-Echeverz J, Duffy AG, Greten TF . The yin and yang of evasion and immune activation in HCC. J Hepatol 2015; 62: 1420–1429.
Gabrilovich DI, Nagaraj S . Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009; 9: 162–174.
Ostrand-Rosenberg S, Sinha P . Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol 2009; 182: 4499–4506.
Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology 2008; 135: 234–243.
Arihara F, Mizukoshi E, Kitahara M, Takata Y, Arai K, Yamashita T et al. Increase in CD14+HLA-DR -/low myeloid-derived suppressor cells in hepatocellular carcinoma patients and its impact on prognosis. Cancer Immunol Immunother 2013; 62: 1421–1430.
Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H et al. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology 2009; 50: 799–807.
Condamine T, Gabrilovich DI . Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol 2011; 32: 19–25.
Zhu AX, Duda DG, Sahani DV, Jain RK . HCC and angiogenesis: possible targets and future directions. Nat Rev Clin Oncol 2011; 8: 292–301.
Wong VW, Yu J, Cheng AS, Wong GL, Chan HY, Chu ES et al. High serum interleukin-6 level predicts future hepatocellular carcinoma development in patients with chronic hepatitis B. Int J Cancer 2009; 124: 2766–2770.
Su S, Liu Q, Chen J, Chen F, He C, Huang D et al. A positive feedback loop between mesenchymal-like cancer cells and macrophages is essential to breast cancer metastasis. Cancer Cell 2014; 25: 605–620.
Dolcetti L, Peranzoni E, Ugel S, Marigo I, Fernandez Gomez A, Mesa C et al. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur J Immunol 2010; 40: 22–35.
Chen MF, Kuan FC, Yen TC, Lu MS, Lin PY, Chung YH et al. IL-6-stimulated CD11b+ CD14+ HLA-DR- myeloid-derived suppressor cells, are associated with progression and poor prognosis in squamous cell carcinoma of the esophagus. Oncotarget 2014; 5: 8716–8728.
Wittamer V, Franssen JD, Vulcano M, Mirjolet JF, Le Poul E, Migeotte I et al. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med 2003; 198: 977–985.
Goralski KB, McCarthy TC, Hanniman EA, Zabel BA, Butcher EC, Parlee SD et al. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem 2007; 282: 28175–28188.
Parolini S, Santoro A, Marcenaro E, Luini W, Massardi L, Facchetti F et al. The role of chemerin in the colocalization of NK and dendritic cell subsets into inflamed tissues. Blood 2007; 109: 3625–3632.
Albanesi C, Scarponi C, Pallotta S, Daniele R, Bosisio D, Madonna S et al. Chemerin expression marks early psoriatic skin lesions and correlates with plasmacytoid dendritic cell recruitment. J Exp Med 2009; 206: 249–258.
Monnier J, Lewen S, O'Hara E, Huang K, Tu H, Butcher EC et al. Expression, regulation, and function of atypical chemerin receptor CCRL2 on endothelial cells. J Immunol 2012; 189: 956–967.
Kaur J, Adya R, Tan BK, Chen J, Randeva HS . Identification of chemerin receptor (ChemR23) in human endothelial cells: chemerin-induced endothelial angiogenesis. Biochem Biophys Res Commun 2010; 391: 1762–1768.
Zabel BA, Nakae S, Zuniga L, Kim JY, Ohyama T, Alt C et al. Mast cell-expressed orphan receptor CCRL2 binds chemerin and is required for optimal induction of IgE-mediated passive cutaneous anaphylaxis. J Exp Med 2008; 205: 2207–2220.
Bondue B, Wittamer V, Parmentier M . Chemerin and its receptors in leukocyte trafficking, inflammation and metabolism. Cytokine Growth Factor Rev 2011; 22: 331–338.
Zhao L, Yang W, Yang X, Lin Y, Lv J, Dou X et al. Chemerin suppresses murine allergic asthma by inhibiting CCL2 production and subsequent airway recruitment of inflammatory dendritic cells. Allergy 2014; 69: 763–774.
Imai K, Takai K, Hanai T, Shiraki M, Suzuki Y, Hayashi H et al. Impact of serum chemerin levels on liver functional reserves and platelet counts in patients with hepatocellular carcinoma. Int J Mol Sci 2014; 15: 11294–11306.
Lin W, Chen YL, Jiang L, Chen JK . Reduced expression of chemerin is associated with a poor prognosis and a lowed infiltration of both dendritic cells and natural killer cells in human hepatocellular carcinoma. Clin Lab 2011; 57: 879–885.
Pachynski RK, Zabel BA, Kohrt HE, Tejeda NM, Monnier J, Swanson CD et al. The chemoattractant chemerin suppresses melanoma by recruiting natural killer cell antitumor defenses. J Exp Med 2012; 209: 1427–1435.
Youn JI, Nagaraj S, Collazo M, Gabrilovich DI . Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol 2008; 181: 5791–5802.
Murdoch C, Muthana M, Coffelt SB, Lewis CE . The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer 2008; 8: 618–631.
He G, Karin M . NF-kappaB and STAT3 - key players in liver inflammation and cancer. Cell Res 2011; 21: 159–168.
Park BK, Zhang H, Zeng Q, Dai J, Keller ET, Giordano T et al. NF-kappaB in breast cancer cells promotes osteolytic bone metastasis by inducing osteoclastogenesis via GM-CSF. Nat Med 2007; 13: 62–69.
Barnea G, Strapps W, Herrada G, Berman Y, Ong J, Kloss B et al. The genetic design of signaling cascades to record receptor activation. Proc Natl Acad Sci USA 2008; 105: 64–69.
Wanninger J, Bauer S, Eisinger K, Weiss TS, Walter R, Hellerbrand C et al. Adiponectin upregulates hepatocyte CMKLR1 which is reduced in human fatty liver. Mol Cell Endocrinol 2012; 349: 248–254.
Fisher DT, Appenheimer MM, Evans SS . The two faces of IL-6 in the tumor microenvironment. Semin Immunol 2014; 26: 38–47.
Teijaro JR, Walsh KB, Cahalan S, Fremgen DM, Roberts E, Scott F et al. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell 2011; 146: 980–991.
Bondue B, Vosters O, de Nadai P, Glineur S, De Henau O, Luangsay S et al. ChemR23 dampens lung inflammation and enhances anti-viral immunity in a mouse model of acute viral pneumonia. PLoS Pathog 2011; 7: e1002358.
Yang S, Wang B, Humphries F, Hogan AE, O'Shea D, Moynagh PN . The E3 ubiquitin ligase Pellino3 protects against obesity-induced inflammation and insulin resistance. Immunity 2014; 41: 973–987.
Tsuyada A, Chow A, Wu J, Somlo G, Chu P, Loera S et al. CCL2 mediates cross-talk between cancer cells and stromal fibroblasts that regulates breast cancer stem cells. Cancer Res 2012; 72: 2768–2779.
Tian Z, Chen Y, Gao B . Natural killer cells in liver disease. Hepatology 2013; 57: 1654–1662.
Condamine T, Ramachandran I, Youn JI, Gabrilovich DI . Regulation of tumor metastasis by myeloid-derived suppressor cells. Annu Rev Med 2015; 66: 97–110.
Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med 2010; 207: 2439–2453.
Lin Y, Yang X, Yue W, Xu X, Li B, Zou L et al. Chemerin aggravates DSS-induced colitis by suppressing M2 macrophage polarization. Cell Mol Immunol 2014; 11: 355–366.
Gao Q, Qiu SJ, Fan J, Zhou J, Wang XY, Xiao YS et al. Intratumoral balance of regulatory and cytotoxic T cells is associated with prognosis of hepatocellular carcinoma after resection. J Clin Oncol 2007; 25: 2586–2593.
Zhang JP, Yan J, Xu J, Pang XH, Chen MS, Li L et al. Increased intratumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J Hepatol 2009; 50: 980–989.
Zhu XD, Zhang JB, Zhuang PY, Zhu HG, Zhang W, Xiong YQ et al. High expression of macrophage colony-stimulating factor in peritumoral liver tissue is associated with poor survival after curative resection of hepatocellular carcinoma. J Clin Oncol 2008; 26: 2707–2716.
Acknowledgements
We thank Dr Yongjun Dang (Department of Biochemistry and Molecular Biology, Fudan University) for the assistance with pulldown assay. This work is supported by National Natural Science Foundation of China grant 813220437, 81471555 (to RH), 31600715 (to YL) and 81272389, 81472674 (to YS).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Information accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Lin, Y., Yang, X., Liu, W. et al. Chemerin has a protective role in hepatocellular carcinoma by inhibiting the expression of IL-6 and GM-CSF and MDSC accumulation. Oncogene 36, 3599–3608 (2017). https://doi.org/10.1038/onc.2016.516
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.516
This article is cited by
-
The chemerin-CMKLR1 axis in keratinocytes impairs innate host defense against cutaneous Staphylococcus aureus infection
Cellular & Molecular Immunology (2024)
-
Chemerin promotes invasion of oral squamous cell carcinoma by stimulating IL-6 and TNF-α production via STAT3 activation
Molecular Biology Reports (2024)
-
Prognostic stratification based on HIF-1α signaling for evaluating hypoxia status and immune landscape in hepatocellular carcinoma
Journal of Big Data (2023)
-
Association of plasma chemerin with all-cause and disease-specific mortality – results from a population-based study
International Journal of Obesity (2023)
-
Small extracellular vesicles as key players in cancer development caused by human oncogenic viruses
Infectious Agents and Cancer (2022)