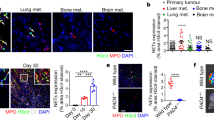
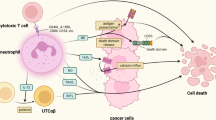
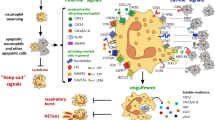
Abstract
Neutrophil extracellular traps (NETs) are a defense mechanism first described to trap and kill bacteria and other pathogens. Increasingly, however, their involvement in the pathogenesis of inflammatory and malignant diseases is being recognized. Several recent studies have suggested important roles of NETs in tumor progression, metastasis and tumor-associated thrombosis. Although systematic studies to address the role of NETs in tumor development are still scarce, we will explore the emerging evidence for NETs as potential protagonists in malignant disease and highlight the mechanisms through which these effects may be exerted. Future questions arising from our current knowledge of direct and indirect interactions between NETs and cancer cells will be outlined and we will explore NETs as candidate pharmaceutical targets in cancer patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Tecchio C, Micheletti A, Cassatella MA . Neutrophil-derived cytokines: facts beyond expression. Front Immunol 2014; 5: 508.
Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS et al. Neutrophil extracellular traps kill bacteria. Science 2004; 303: 1532–1535.
Garcia-Romo GS, Caielli S, Vega B, Connolly J, Allantaz F, Xu Z et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci Transl Med 2011; 3: 73ra20.
Brill A, Fuchs TA, Savchenko AS, Thomas GM, Martinod K, De Meyer SF et al. Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost 2012; 10: 136–144.
Wong SL, Demers M, Martinod K, Gallant M, Wang Y, Goldfine AB et al. Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat Med 2015; 21: 815–819.
Gupta AK, Hasler P, Holzgreve W, Gebhardt S, Hahn S . Induction of neutrophil extracellular DNA lattices by placental microparticles and IL-8 and their presence in preeclampsia. Hum Immunol 2005; 66: 1146–1154.
Sur Chowdhury C, Giaglis S, Walker UA, Buser A, Hahn S, Hasler P . Enhanced neutrophil extracellular trap generation in rheumatoid arthritis: analysis of underlying signal transduction pathways and potential diagnostic utility. Arthritis Res Ther 2014; 16: R122.
Savchenko AS, Borissoff JI, Martinod K, De Meyer SF, Gallant M, Erpenbeck L et al. VWF-mediated leukocyte recruitment with chromatin decondensation by PAD4 increases myocardial ischemia/reperfusion injury in mice. Blood 2014; 123: 141–148.
Pilsczek FH, Salina D, Poon KK, Fahey C, Yipp BG, Sibley CD et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. J Immunol 2010; 185: 7413–7425.
Yousefi S, Mihalache C, Kozlowski E, Schmid I, Simon HU . Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ 2009; 16: 1438–1444.
Yipp BG, Kubes P . NETosis: how vital is it? Blood 2013; 122: 2784–2794.
Yipp BG, Petri B, Salina D, Jenne CN, Scott BN, Zbytnuik LD et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat Med 2012; 18: 1386–1393.
Urban CF, Reichard U, Brinkmann V, Zychlinsky A . Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol 2006; 8: 668–676.
Raftery MJ, Lalwani P, Krautkrmer E, Peters T, Scharffetter-Kochanek K, Kruger R et al. beta2 integrin mediates hantavirus-induced release of neutrophil extracellular traps. J Exp Med 2014; 211: 1485–1497.
Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med 2007; 13: 463–469.
Ben-Smith A, Dove SK, Martin A, Wakelam MJ, Savage CO . Antineutrophil cytoplasm autoantibodies from patients with systemic vasculitis activate neutrophils through distinct signaling cascades: comparison with conventional Fcgamma receptor ligation. Blood 2001; 98: 1448–1455.
Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V et al. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol 2007; 176: 231–241.
Parker H, Dragunow M, Hampton MB, Kettle AJ, Winterbourn CC . Requirements for NADPH oxidase and myeloperoxidase in neutrophil extracellular trap formation differ depending on the stimulus. J Leukoc Biol 2012; 92: 841–849.
Douda DN, Khan MA, Grasemann H, Palaniyar N . SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc Natl Acad Sci USA 2015; 112: 2817–2822.
Lewis HD, Liddle J, Coote JE, Atkinson SJ, Barker MD, Bax BD et al. Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat Chem Biol 2015; 11: 189–191.
Metzler KD, Goosmann C, Lubojemska A, Zychlinsky A, Papayannopoulos V . A myeloperoxidase-containing complex regulates neutrophil elastase release and actin dynamics during NETosis. Cell Rep 2014; 8: 883–896.
Martinod K, Witsch T, Farley K, Gallant M, Remold-O'Donnell E, Wagner DD . Neutrophil elastase-deficient mice form neutrophil extracellular traps in an experimental model of deep vein thrombosis. J Thromb Haemost 2015; 14: 551–558.
Schauer C, Janko C, Munoz LE, Zhao Y, Kienhofer D, Frey B et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat Med 2014; 20: 511–517.
Gupta AK, Joshi MB, Philippova M, Erne P, Hasler P, Hahn S et al. Activated endothelial cells induce neutrophil extracellular traps and are susceptible to NETosis-mediated cell death. FEBS Lett 2010; 584: 3193–3197.
Demers M, Krause DS, Schatzberg D, Martinod K, Voorhees JR, Fuchs TA et al. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc Natl Acad Sci U S A 2012; 109: 13076–13081.
Bodey B, Bodey B Jr, Siegel SE, Luck JV, Kaiser HE . Immunophenotypic characterization of human primary and metastatic melanoma infiltrating leukocytes. Anticancer Res 1996; 16: 3439–3446.
Houghton AM . The paradox of tumor-associated neutrophils: fueling tumor growth with cytotoxic substances. Cell Cycle 2014; 9: 1732–1737.
Houghton AM . The paradox of tumor-associated neutrophils: fueling tumor growth with cytotoxic substances. Cell Cycle 2010; 9: 1732–1737.
Hu DE, Hori Y, Fan TP . Interleukin-8 stimulates angiogenesis in rats. Inflammation 1993; 17: 135–143.
Jaillon S, Peri G, Delneste Y, Fremaux I, Doni A, Moalli F et al. The humoral pattern recognition receptor PTX3 is stored in neutrophil granules and localizes in extracellular traps. J Exp Med 2007; 204: 793–804.
An H, Zhu Y, Xie H, Liu Y, Liu W, Fu Q et al. Increased expression of interleukin-8 is an independent indicator of poor prognosis in clear-cell renal cell carcinoma. Tumour Biol 2015; 37: 4523–4529.
Berger-Achituv S, Brinkmann V, Abed UA, Kuhn LI, Ben-Ezra J, Elhasid R et al. A proposed role for neutrophil extracellular traps in cancer immunoediting. Front Immunol 2013; 4: 48.
Zhao JJ, Pan K, Wang W, Chen JG, Wu YH, Lv L et al. The prognostic value of tumor-infiltrating neutrophils in gastric adenocarcinoma after resection. PloS One 2012; 7: e33655.
Perisanidis C, Kornek G, Poschl PW, Holzinger D, Pirklbauer K, Schopper C et al. High neutrophil-to-lymphocyte ratio is an independent marker of poor disease-specific survival in patients with oral cancer. Med Oncol 2013; 30: 334.
Xiao WK, Chen D, Li SQ, Fu SJ, Peng BG, Liang LJ . Prognostic significance of neutrophil-lymphocyte ratio in hepatocellular carcinoma: a meta-analysis. BMC Cancer 2014; 14: 117.
Ho-Tin-Noe B, Carbo C, Demers M, Cifuni SM, Goerge T, Wagner DD . Innate immune cells induce hemorrhage in tumors during thrombocytopenia. Am J Pathol 2009; 175: 1699–1708.
Thomas MP, Whangbo J, McCrossan G, Deutsch A, Martinod K, Walch M et al. Leukocyte protease binding to nucleic acids promotes nuclear localization and cleavage of nucleic acid binding proteins. J Immunol 2014; 192: 5390–5397.
Kolaczkowska E, Jenne CN, Surewaard BG, Thanabalasuriar A, Lee WY, Sanz MJ et al. Molecular mechanisms of NET formation and degradation revealed by intravital imaging in the liver vasculature. Nat Commun 2015; 6: 6673.
Weinrauch Y, Drujan D, Shapiro SD, Weiss J, Zychlinsky A . Neutrophil elastase targets virulence factors of enterobacteria. Nature 2002; 417: 91–94.
Houghton AM, Rzymkiewicz DM, Ji H, Gregory AD, Egea EE, Metz HE et al. Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat Med 2010; 16: 219–223.
Sangaletti S, Tripodo C, Vitali C, Portararo P, Guarnotta C, Casalini P et al. Defective stromal remodeling and neutrophil extracellular traps in lymphoid tissues favor the transition from autoimmunity to lymphoma. Cancer Disc 2014; 4: 110–129.
Wada Y, Yoshida K, Tsutani Y, Shigematsu H, Oeda M, Sanada Y et al. Neutrophil elastase induces cell proliferation and migration by the release of TGF-alpha, PDGF and VEGF in esophageal cell lines. Oncol Rep 2007; 17: 161–167.
Gaida MM, Steffen TG, Gunther F, Tschaharganeh DF, Felix K, Bergmann F et al. Polymorphonuclear neutrophils promote dyshesion of tumor cells and elastase-mediated degradation of E-cadherin in pancreatic tumors. Eur J Immunol 2012; 42: 3369–3380.
Heissig B, Nishida C, Tashiro Y, Sato Y, Ishihara M, Ohki M et al. Role of neutrophil-derived matrix metalloproteinase-9 in tissue regeneration. Histol Histopathol 2010; 25: 765–770.
Martinod K, Fuchs TA, Zitomersky NL, Wong SL, Demers M, Gallant M et al. PAD4-deficiency does not affect bacteremia in polymicrobial sepsis and ameliorates endotoxemic shock. Blood 2015; 125: 1948–1956.
Acuff HB, Carter KJ, Fingleton B, Gorden DL, Matrisian LM . Matrix metalloproteinase-9 from bone marrow-derived cells contributes to survival but not growth of tumor cells in the lung microenvironment. Cancer Res 2006; 66: 259–266.
Coussens LM, Tinkle CL, Hanahan D, Werb Z . MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell 2000; 103: 481–490.
Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K et al. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol 2000; 2: 737–744.
Nozawa H, Chiu C, Hanahan D . Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci U S A 2006; 103: 12493–12498.
Wang J, Wang Y, Wang S, Cai J, Shi J, Sui X et al. Bone marrow-derived mesenchymal stem cell-secreted IL-8 promotes the angiogenesis and growth of colorectal cancer. Oncotarget 2015; 6: 42825–42837.
Smith DR, Polverini PJ, Kunkel SL, Orringer MB, Whyte RI, Burdick MD et al. Inhibition of interleukin 8 attenuates angiogenesis in bronchogenic carcinoma. J Exp Med 1994; 179: 1409–1415.
Bald T, Quast T, Landsberg J, Rogava M, Glodde N, Lopez-Ramos D et al. Ultraviolet-radiation-induced inflammation promotes angiotropism and metastasis in melanoma. Nature 2014; 507: 109–113.
Demers M, Wong SL, Martinod K, Gallant M, Cabral JE, Wang Y et al. Priming of neutrophils toward NETosis promotes tumor growth. Oncoimmunology 2016; 5: e1134073.
Varki A . Trousseau’s syndrome: multiple definitions and multiple mechanisms. Blood 2007; 110: 1723–1729.
Erpenbeck L, Nieswandt B, Schon M, Pozgajova M, Schon MP . Inhibition of platelet GPIb alpha and promotion of melanoma metastasis. J Investig Dermatol 2010; 130: 576–586.
Fuchs TA, Brill A, Wagner DD . Neutrophil extracellular trap (NET) impact on deep vein thrombosis. Arterioscler Thromb Vasc Biol 2012; 32: 1777–1783.
Massberg S, Grahl L, von Bruehl ML, Manukyan D, Pfeiler S, Goosmann C et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med 2010; 16: 887–896.
von Bruhl ML, Stark K, Steinhart A, Chandraratne S, Konrad I, Lorenz M et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med 2012; 209: 819–835.
Ammollo CT, Semeraro F, Xu J, Esmon NL, Esmon CT . Extracellular histones increase plasma thrombin generation by impairing thrombomodulin-dependent protein C activation. J Thromb Haemost 2011; 9: 1795–1803.
Semeraro F, Ammollo CT, Morrissey JH, Dale GL, Friese P, Esmon NL et al. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood 2011; 118: 1952–1961.
Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD Jr. et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A 2010; 107: 15880–15885.
Geddings JE, Mackman N . Tumor-derived tissue factor-positive microparticles and venous thrombosis in cancer patients. Blood 2013; 122: 1873–1880.
Thomas GM, Brill A, Mezouar S, Crescence L, Gallant M, Dubois C et al. Tissue factor expressed by circulating cancer cell-derived microparticles drastically increases the incidence of deep vein thrombosis in mice. J Thromb Haemost 2015; 13: 1310–1319.
Alfaro C, Teijeira A, Onate C, Perez G, Sanmamed MF, Andueza MP et al. Tumor-produced interleukin-8 attracts human myeloid-derived suppressor cells and elicits extrusion of neutrophil extracellular traps (NETs). Clin Cancer Res 2016; 22: 3924–3936.
Baj-Krzyworzeka M, Baran J, Weglarczyk K, Szatanek R, Szaflarska A, Siedlar M et al. Tumour-derived microvesicles (TMV) mimic the effect of tumour cells on monocyte subpopulations. Anticancer Res 2010; 30: 3515–3519.
Keshari RS, Jyoti A, Dubey M, Kothari N, Kohli M, Bogra J et al. Cytokines induced neutrophil extracellular traps formation: implication for the inflammatory disease condition. PloS One 2012; 7: e48111.
Carmona-Rivera C, Kaplan MJ . Low-density granulocytes: a distinct class of neutrophils in systemic autoimmunity. Semin Immunopathol 2013; 35: 455–463.
Knight JS, Carmona-Rivera C, Kaplan MJ . Proteins derived from neutrophil extracellular traps may serve as self-antigens and mediate organ damage in autoimmune diseases. Front Immunol 2012; 3: 380.
Guglietta S, Chiavelli A, Zagato E, Krieg C, Gandini S, Ravenda PS et al. Coagulation induced by C3aR-dependent NETosis drives protumorigenic neutrophils during small intestinal tumorigenesis. Nat Commun 2016; 7: 11037.
Terraube V, Marx I, Denis CV . Role of von Willebrand factor in tumor metastasis. Thromb Res 2007; 120 (Suppl 2): S64–S70.
Franchini M, Frattini F, Crestani S, Bonfanti C, Lippi G . von Willebrand factor and cancer: a renewed interest. Thromb Res 2013; 131: 290–292.
Starke RD, Ferraro F, Paschalaki KE, Dryden NH, McKinnon TA, Sutton RE et al. Endothelial von Willebrand factor regulates angiogenesis. Blood 2011; 117: 1071–1080.
Bauer AT, Suckau J, Frank K, Desch A, Goertz L, Wagner AH et al. von Willebrand factor fibers promote cancer-associated platelet aggregation in malignant melanoma of mice and humans. Blood 2015; 125: 3153–3163.
Martinod K, Demers M, Fuchs TA, Wong SL, Brill A, Gallant M et al. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc Natl Acad Sci USA 2013; 110: 8674–8679.
Grassle S, Huck V, Pappelbaum KI, Gorzelanny C, Aponte-Santamaria C, Baldauf C et al. von Willebrand factor directly interacts with DNA from neutrophil extracellular traps. Arterioscler Thromb Vasc Biol 2014; 34: 1382–1389.
Ludwig RJ, Boehme B, Podda M, Henschler R, Jager E, Tandi C et al. Endothelial P-selectin as a target of heparin action in experimental melanoma lung metastasis. Cancer Res 2004; 64: 2743–2750.
Etulain J, Martinod K, Wong SL, Cifuni SM, Schattner M, Wagner DD . P-selectin promotes neutrophil extracellular trap formation in mice. Blood 2015; 126: 242–246.
McDonald B, Spicer J, Giannais B, Fallavollita L, Brodt P, Ferri LE . Systemic inflammation increases cancer cell adhesion to hepatic sinusoids by neutrophil mediated mechanisms. Int J Cancer 2009; 125: 1298–1305.
Spicer JD, McDonald B, Cools-Lartigue JJ, Chow SC, Giannias B, Kubes P et al. Neutrophils promote liver metastasis via Mac-1-mediated interactions with circulating tumor cells. Cancer Res 2012; 72: 3919–3927.
Liang S, Hoskins M, Khanna P, Kunz RF, Dong C . Effects of the tumor-leukocyte microenvironment on melanoma-neutrophil adhesion to the endothelium in a shear flow. Cell Mol Bio 2008; 1: 189–200.
Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest 2013.
Cools-Lartigue J, Spicer J, Najmeh S, Ferri L . Neutrophil extracellular traps in cancer progression. Cell Mol Life Sci 2014; 71: 4179–4194.
Kruss S, Erpenbeck L, Amschler K, Mundinger TA, Boehm H, Helms HJ et al. Adhesion maturation of neutrophils on nanoscopically presented platelet glycoprotein Ibalpha. ACS Nano 2013; 7: 9984–9996.
Erpenbeck L, Schon MP . Deadly allies: the fatal interplay between platelets and metastasizing cancer cells. Blood 2010; 115: 3427–3436.
Quan JM, Tiddens HA, Sy JP, McKenzie SG, Montgomery MD, Robinson PJ et al. A two-year randomized, placebo-controlled trial of dornase alfa in young patients with cystic fibrosis with mild lung function abnormalities. Journal Pediatr 2001; 139: 813–820.
Sugihara S, Yamamoto T, Tanaka H, Kambara T, Hiraoka T, Miyauchi Y . Deoxyribonuclease treatment prevents blood-borne liver metastasis of cutaneously transplanted tumour cells in mice. Br J Cancer 1993; 67: 66–70.
Cedervall J, Zhang Y, Huang H, Zhang L, Femel J, Dimberg A et al. Neutrophil extracellular traps accumulate in peripheral blood vessels and compromise organ function in tumor-bearing animals. Cancer Res 2015; 75: 2653–2662.
Lin AM, Rubin CJ, Khandpur R, Wang JY, Riblett M, Yalavarthi S et al. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J Immunol 2011; 187: 490–500.
Lande R, Ganguly D, Facchinetti V, Frasca L, Conrad C, Gregorio J et al. Neutrophils activate plasmacytoid dendritic cells by releasing self-DNA-peptide complexes in systemic lupus erythematosus. Sci Transl Med 2011; 3: 73ra19.
Warnatsch A, Ioannou M, Wang Q, Papayannopoulos V . Inflammation. Neutrophil extracellular traps license macrophages for cytokine production in atherosclerosis. Science 2015; 349: 316–320.
Sangaletti S, Tripodo C, Chiodoni C, Guarnotta C, Cappetti B, Casalini P et al. Neutrophil extracellular traps mediate transfer of cytoplasmic neutrophil antigens to myeloid dendritic cells toward ANCA induction and associated autoimmunity. Blood 2012; 120: 3007–3018.
Amschler K, Erpenbeck L, Kruss S, Schon MP . Nanoscale integrin ligand patterns determine melanoma cell behavior. ACS Nano 2014; 8: 9113–9125.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Erpenbeck, L., Schön, M. Neutrophil extracellular traps: protagonists of cancer progression?. Oncogene 36, 2483–2490 (2017). https://doi.org/10.1038/onc.2016.406
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2016.406
This article is cited by
-
Dormancy of cutaneous melanoma
Cancer Cell International (2024)
-
Neutrophil extracellular trap formation during surgical procedures: a pilot study
Scientific Reports (2023)
-
Recent advances in the role of neutrophils and neutrophil extracellular traps in acute pancreatitis
Clinical and Experimental Medicine (2023)
-
Neutrophil extracellular traps have auto-catabolic activity and produce mononucleosome-associated circulating DNA
Genome Medicine (2022)
-
Gut microbiota influence immunotherapy responses: mechanisms and therapeutic strategies
Journal of Hematology & Oncology (2022)